Respiratory tract infections are common in children. Evidence for the efficacy of over‐the‐counter cough and cold medications in young children is limited,1,2 but they can lead to severe adverse events, including death.3 In Australia, the Therapeutic Goods Administration (TGA) introduced compulsory labelling changes for non‐prescription cough and cold products in 2012 (but not herbal products),4 with further changes in 2020 for first generation (sedating) antihistamine‐containing products5 (further details: ).
We evaluated whether these labelling changes were followed by reductions in reported poisoning exposures. We reviewed calls to the New South Wales Poisons Information Centre (NSWPIC) during 1 January 2010 – 31 December 2021 about children under six years of age exposed to over‐the‐counter cough and cold medications. NSWPIC receives about 50% of the approximately 220 000 calls to Australian poisons information centres each year. We estimated annual percentage changes (APCs) in annual report rate with the regression program, Joinpoint 4.8.0.1, using permutation test model selection (). The study was approved by the Sydney Children's Hospitals Network Human Research Ethics Committee (2021/ETH00165).
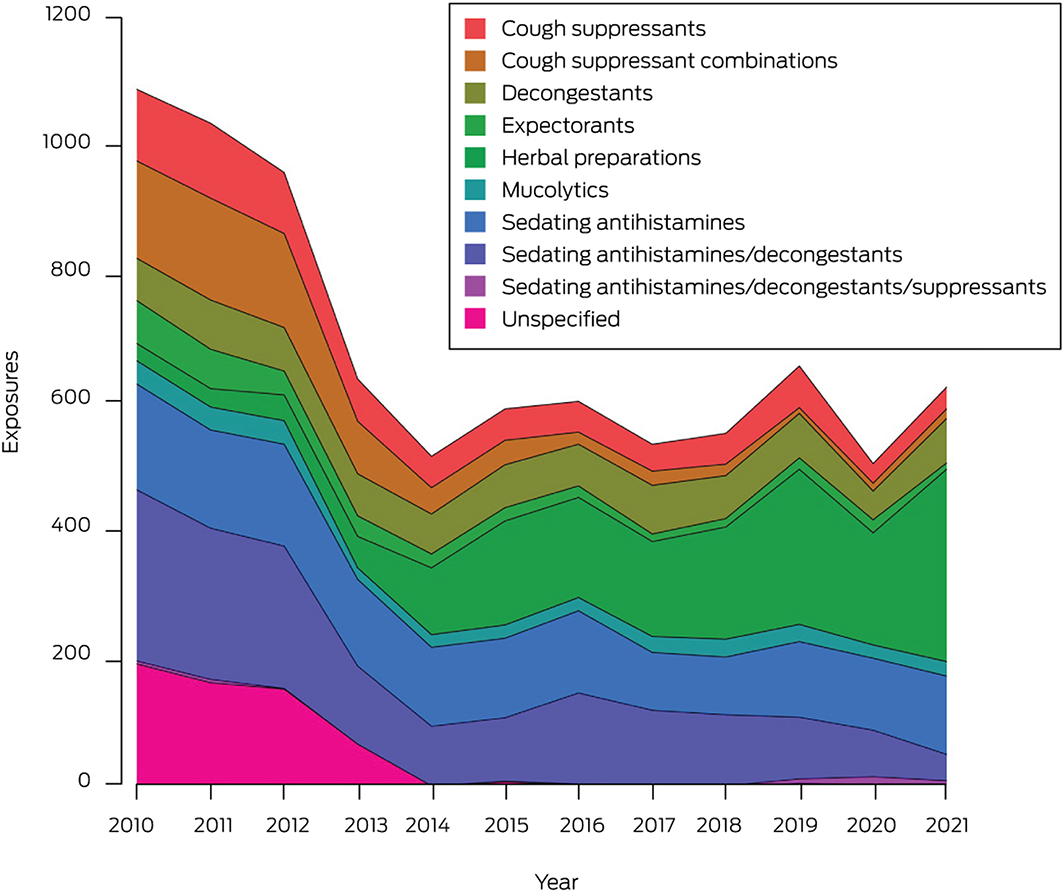
A total of 8327 exposures of children under six years of age to non‐prescription cough and cold products were reported during 2010–2021 (Box). Almost all were accidental exposures (4264, 51.2%) or therapeutic errors (3953, 47.5%) (Supporting Information, table 2). The mean annual number of reported exposures declined significantly, from 1067 before to 587 after the 2012 changes (APC, –6.3%; 95% confidence interval [CI], –10.3% to –2.0%), primarily because of the decline during 2010–2014 (APC, –17.3%; 95% CI, –26.5% to –6.9%). For products subject to the 2012 labelling change (ie, non‐herbal preparations only), the declines were more marked (2010–2021: APC, –11.1%; 95% CI, –14.5% to –7.5%; 2010–2014: –21.4%; 95% CI, –29.2% to –12.7%). The largest decline was for exposures to combinations of sedating antihistamines and decongestants (APC, –15.8%; 95% CI, –26.2% to –3.8%). Exposures to formulations of sedating antihistamines without decongestants did not decline significantly across the entire study period, nor after the 2020 labelling changes, although a reduction during 2010–2018 was evident (APC, –6.8%; 95% CI, –9.8% to –3.8%). Reported exposures to herbal preparations increased during 2010–2021 (APC, 25.1%; 95% CI, 16.0–34.9%) (Supporting Information, table 3, figure 1).
During the 2018 cold and influenza season (1 April – 31 October), off‐label medication use was involved in 92 of 250 calls (37%) for which this information could be collected, including 54 for brompheniramine/phenylephrine combinations. Off‐label use was recommended by health care professionals in 39 of the 53 cases for which this information was recorded (74%).
The proportions of children exposed to non‐prescription cough and cold products who required hospital management were similar before (334 of 2485, 11.7%) and after August 2012 (686 of 5482, 12.5%), but the mean annual number declined from 125.3 to 73.5 children.
In conclusion, we found that reported poisonings of children under six years of age with non‐prescription cough and cold products have declined since the 2012 labelling changes. In the United States, a substantial decline in the numbers of accidental ingestions and therapeutic errors in children under six years of age followed a similar intervention.6 Health care professional and public education campaigns discouraging the use of non‐prescription cough and cold products may have played a role in reducing the number of poisonings in NSW children. Nevertheless, about 600 exposures of children under six, including 75 who require hospital management, are still reported to NSWPIC each year. Further, many herbal products were launched after 2012 as they could be labelled as suitable for young children. These products are generally safe and overdose does not typically cause toxicity, but evidence for their efficacy is very limited.7
The continued off‐label use of products for treating coughs and colds in young children suggests that health care professionals and the public may underappreciate their risks. This indicates that further education and clinical guidance are needed.
Received 15 November 2022, accepted 7 February 2023
- 1. Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database Syst Rev 2014; CD001831.
- 2. Vassilev ZP, Kabadi S, Villa R. Safety and efficacy of over‐the‐counter cough and cold medicines for use in children. Expert Opin Drug Saf 2010; 9: 233‐242.
- 3. Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med 2009; 53: 411‐417.
- 4. Therapeutic Goods Administration. OTC cough and cold medicines for children: final outcomes of TGA review. 15 Aug 2012. Archived: https://web.archive.org/web/20140717013231/http://tga.gov.au/industry/otc‐notices‐cough‐cold‐review‐outcomes.htm#.U8cntK3P0Q8 (viewed Feb 2023).
- 5. Therapeutic Goods Administration. First‐generation oral sedating antihistamines: do not use in children [media release]. 13 July 2022. https://www.tga.gov.au/news/safety‐updates/first‐generation‐oral‐sedating‐antihistamines‐do‐not‐use‐children (viewed Feb 2023).
- 6. Mazer‐Amirshahi M, Reid N, van den Anker J, Litovitz T. Effect of cough and cold medication restriction and label changes on pediatric ingestions reported to United States poison centers. J Pediat 2013; 163: 1372‐1376.
- 7. Sierocinski E, Holzinger F, Chenot JF. Ivy leaf (Hedera helix) for acute upper respiratory tract infections: an updated systematic review. Eur J Clin Pharmacol 2021; 77: 1113‐1122.






Open access
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
We thank staff at the NSW Poisons Information Centre. Rose Cairns is supported by a National Health and Medical Research Council Investigator Grant (1196516). Abrar Arbaeen holds a Saudi Arabian Ministry of Education scholarship.
Rose Cairns has an untied educational grant from Reckitt to fund doctoral research concerning over‐the‐counter analgesics, unrelated to the content of this article.