Recent advances in critical care relevant to a broad range of clinicians
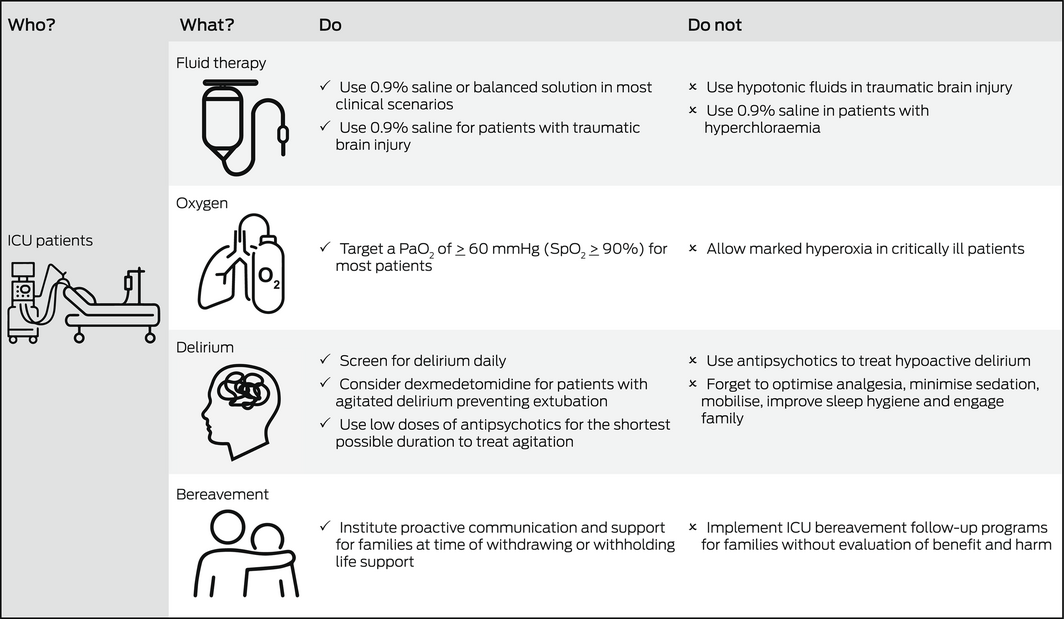
Global interest in the management of critically ill patients has increased significantly with the coronavirus disease 2019 (COVID‐19) pandemic. This Perspective article focuses on recent advances in four key aspects of critical care that are relevant to a broad range of clinicians including those who do not practise in an intensive care unit (ICU): intravenous fluid therapy, supplemental oxygen, management of delirium, and follow‐up care of bereaved family members (Box).
Fluid therapy
Fluid therapy is ubiquitous in ICU. Thus, even small differences in clinical outcomes according to the type of fluid have significant repercussions for patients. Previous large randomised studies comparing albumin with crystalloid solutions failed to show differences in clinical outcomes, such as overall mortality. However, if crystalloids are to be used, clinicians need to decide whether to use saline or a balanced solution. Balanced solutions (Hartmann's solution or Plasma‐Lyte in Australia) have a sodium, potassium and chloride content similar to that of extracellular fluid and may have lesser impact on acid‐base balance. This issue has been recently studied by the Balanced Solution versus Saline in Intensive Care Study (BaSICS) trial,1 which compared normal saline to Plasma‐Lyte. The study found no overall difference in 90‐day mortality or in any other clinical outcomes. However, BaSICS also found a significant 48% relative increase in mortality among patients with traumatic brain injury (TBI) who received Plasma‐Lyte (the lower tonicity fluid). The importance of avoiding hypotonic fluids in patients with TBI had been previously noted; thus, saline is the preferred fluid in such patients.2 Of relevance to clinicians working in the pre‐hospital or emergency setting, a post hoc analysis of the BaSICS study3 found a high probability that balanced solution use was associated with lower 90‐day mortality in critically ill patients who exclusively received balanced solutions before study enrolment. This benefit was most evident in patients with unplanned admission due to sepsis. In addition, BaSICS compared a fluid bolus administration rate of 333 mL/h versus 999 mL/h (but similar fluid volumes). The investigators reported that speed of delivery did not affect outcomes.4 The publication of BaSICS has recently been followed by the publication of a complementary randomised double‐blinded trial, the Plasma‐Lyte 148 versus Saline (PLUS) study, which did not identify a difference between the two fluids (PLUS excluded patients with TBI).5 Data collection for BaSICS and PLUS was harmonised and the individual patient data meta‐analysis from these trials will provide important insights.
The Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care (CLASSIC) trial compared a restrictive versus usual care fluid therapy regimen in 1550 adults with septic shock.6 The median cumulative volume of intravenous fluids in ICU was 2 L less with a restrictive regimen. However, intravenous fluid restriction did not result in lower 90‐day mortality, serious adverse events, or use of renal replacement therapy. Of note, most patients in the CLASSIC trial had a gastrointestinal source of infection and may have required large volumes of replacement fluid if diarrhoea or vomiting were prominent. Similarly, the findings of the CLASSIC trial may not apply to patients without sepsis (eg, critically ill postoperative patients). A number of ongoing large trials (CLOVERS, Clinicaltrials.gov: NCT03434028; ARISE‐FLUIDS, Clinicaltrials.gov: NCT04569942; and EVIS, Clinicaltrials.gov: NCT05179499) will clarify any potential benefits of restrictive fluid strategies and early vasopressor use in sepsis.
Of note, a recent multicentre randomised trial of older patients (aged ≥ 65 years) with vasodilatory hypotension — most of whom had sepsis — compared the effect of reducing exposure to vasopressors through permissive hypotension (mean arterial pressure target of 60–65 mmHg) with usual care.7 There was no clinically important difference in fluid balance between groups, and permissive hypotension did not significantly reduce mortality at 90 days.
Therefore, it is logical that physiological considerations may currently play a role in the choice of intravenous fluid in different clinical situations (eg, hyperchloraemia, metabolic acidosis, acute kidney injury, high risk of cerebral oedema) where saline or balanced fluid may be more physiologically justified. Further evidence is required to support a restrictive approach to fluid therapy or blood pressure targets in sepsis.
Supplemental oxygen
Many hospitalised patients receive supplemental oxygen, and the impact of small differences in outcomes according to oxygen dose is just as important as described above for fluid therapy. Administration of a greater fraction of inspired oxygen (FiO2) frequently causes the partial pressure of oxygen in arterial blood (PaO2) to be greater than normal (hyperoxia). In humans, hyperoxia has marked physiological effects and strong associations with adverse outcomes, including increased mortality.8 Recent randomised ICU trials have attempted to understand whether strategies to avoid hyperoxia improve outcomes.
The Intensive Care Unit Randomized Trial Comparing Two Approaches to Oxygen Therapy (ICU‐ROX) trial, which included 21 ICUs and 1000 ventilated patients in New Zealand and Australia,9 randomly assigned patients to conservative oxygen or usual oxygen therapy. In the conservative oxygen group, the upper alarm limit of the pulse oximetry oxygen saturation (SpO2) was set at 97% and the FiO2 decreased to 0.21 if the SpO2 was above 90%. In the usual oxygen group, there were no specific measures to limit the FiO2 or the SpO2. The conservative oxygen group received less FiO2 and had lower SpO2. However, there were no differences in days of ventilation or mortality.
In the lower or higher oxygen targets for acute hypoxaemic respiratory failure (HOT‐ICU) trial, in 35 European ICUs,10 2928 patients with acute hypoxaemic respiratory failure receiving ≥ 10 L of oxygen per minute in an open system or a FiO2 ≥ 0.5 in a closed system were assigned to a lower oxygenation group (target PaO2 60 mmHg) or a higher oxygenation group (target PaO2 90 mmHg). There was no significant difference in outcomes.
The liberal or conservative oxygen therapy for acute respiratory distress syndrome (LOCO2) trial, in 13 ICUs in France,11 randomly assigned 205 ventilated patients with acute respiratory distress syndrome to conservative oxygen therapy (target PaO2 55–70 mmHg or SpO2 88–92%) or liberal oxygen therapy (target PaO2 90–105 mmHg or SpO2 ≥ 96%). The investigators stopped the trial early, leading to a small sample size, because of the potential for harm, with five events of mesenteric ischaemia in the conservative oxygen group. Clinical outcomes, including the primary outcome of day 28 mortality, were not statistically different but there were more deaths by day 90 in patients assigned to conservative oxygen therapy (44/99 v 31/102 patients; difference, 14.0 percentage points; 95% CI, 0.7–27.2). The meaning of this safety signal to clinical practice is unclear.
Importantly, these trials did not test the effect of marked hyperoxia, and the number of patients does not exclude a 1–2% difference in mortality or longer term neurocognitive effect. Clinicians caring for hospitalised patients should be aware that existing evidence supports neither a liberal nor conservative oxygen strategy. However, given the risks of oxygen toxicity, marked hyperoxia (PaO2 > 300 mmHg) should be avoided.8,12
Delirium
Delirium occurs frequently in the ICU, affecting up to 75% of mechanically ventilated patients,13 and is associated with increased mortality, longer periods of hospitalisation, and increased costs. Furthermore, ICU patients who experience delirium are more likely to have long term cognitive impairment comparable to moderate TBI or mild Alzheimer disease at 12 months after ICU discharge.13 Although the importance of recognising and treating delirium is well established, effective strategies to do so remain limited.
Despite their widespread use, there is a lack of evidence that antipsychotic medications improve brain function in delirious ICU patients. The Modifying the Impact of the ICU‐Associated Neurological Dysfunction‐USA (MIND‐USA) trial,14 the largest recent blinded randomised controlled trial of antipsychotics in critical illness, enrolled 566 delirious ICU patients in the United States. Participants were randomly assigned to the typical antipsychotic haloperidol, the atypical antipsychotic ziprasidone, or to placebo given intravenously. The use of haloperidol or ziprasidone, when compared with placebo, did not alter the number of days alive and without delirium or coma during the 14‐day intervention period. It is important to note, however, that most patients in the MIND‐USA trial had hypoactive delirium. If other measures have failed, antipsychotics may be necessary to treat complications of hyperactive delirium, including aggression and removal of ICU devices (eg, endotracheal tube for ventilation or vascular access devices) by the patient, ideally in the smallest doses and shortest duration necessary.
Smaller studies have examined medications other than antipsychotics. A randomised blinded trial of 142 patients found that high dose simvastatin does not increase days alive without delirium and coma at day 14 compared with placebo.15 The Dexmedetomidine to Lessen ICU Agitation (DahLIA) trial, conducted in 15 ICUs in Australia and New Zealand, randomly assigned 74 patients deemed too delirious for extubation to the α‐2 agonist dexmedetomidine or placebo.16 Dexmedetomidine increased ventilator‐free hours at seven days (median difference, 17 h; 95% CI, 4–33.2 h). However, this study was terminated early because of lack of trial drug provision.
No pharmacological agents appear to prevent delirium in the ICU. The prophylactic melatonin for delirium in intensive care (Pro‐MEDIC) trial randomly assigned 847 patients in 12 Australian ICUs to melatonin or placebo within 48 hours of admission for 14 consecutive nights.17 Melatonin did not increase the proportion of delirium‐free assessments per patient nor improve sleep.
Given the limited efficacy of pharmacological interventions for delirium, best practice should include optimising analgesia, minimising sedation and non‐pharmacological strategies such as early mobilisation, improving sleep hygiene, correcting visual and hearing impairments, and engaging family. Such interventions may reduce delirium when delivered together as a bundle of care, although the quality of evidence for this is low.18
Bereavement care
Family members of patients who die in the ICU may be at increased risk of complicated grief, anxiety, depression, and post‐traumatic stress disorder (PTSD). Bereavement services have been proposed to support family members. However, interventions such as ICU diaries, mementoes, storytelling, sympathy letters, and follow‐up phone calls and meetings have little evidence of benefit.
In a multicentre randomised trial of 242 relatives of patients who died in French ICUs, a handwritten letter from the treating doctor and nurse actually increased symptoms of depression (36.6% v 24.7%; P = 0.05) and PTSD (52.4% v 37.1%; P = 0.03) at 6 months compared with standard care.19 Similarly, a recent three parallel‐group randomised trial in an Australian ICU found that anxiety, depression and PTSD symptoms in 71 bereaved relatives were not alleviated by either a condolence letter or a telephone call from hospital staff.20
A recent systematic review and meta‐analysis identified significant variability in the design, implementation and assessment of ICU bereavement support programs.21 The meta‐analysis of three studies of written support materials and two studies of narration of relatives’ experiences in the ICU demonstrated no effect on anxiety and depression.
Given such uncertainty, bereavement support interventions should undergo further evaluation before widespread implementation. Furthermore, recent evidence suggests that proactive communication and support interventions before a death in the ICU (at the time of withdrawing or withholding life support) can successfully reduce prolonged grief symptoms in family members22 and this should be the focus of future studies.
Conclusion and future directions
This article has discussed recent advances in critical care which are of importance to a variety of clinicians. The evidence presented has been generated from traditional randomised controlled trials. However, there has been a significant increase in critical care trials using novel designs during the COVID‐19 pandemic. Using a platform framework and response‐adaptive randomisation, several therapies can be evaluated simultaneously and rapidly. The REMAP‐CAP trial recruited thousands of critically ill patients with COVID‐19, including from Australia, and generated important evidence about the role of corticosteroids, anticoagulation, and interleukin‐6 inhibitors using these novel designs.23,24 Adaptive platform trials rapidly inform practice but require complex set‐up and planning, and a relatively stable event rate over time. Researchers and clinicians are still learning how disease variants and institutional strain affect mortality rates over time and how these factors influence results when using response‐adaptive randomisation. The upcoming critical care trial landscape is highly likely to include a mix of conventional and novel trial designs to inform future clinical practice.
Provenance: Commissioned; externally peer reviewed.
- 1. Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BASICS randomized clinical trial. JAMA 2021; 326: 1‐12.
- 2. SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health; Myburgh J, Cooper DJ, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 2007; 357: 874‐884.
- 3. Zampieri FG, Machado FR, Biondi RS, et al. Association between type of fluid received prior to enrollment, type of admission, and effect of balanced crystalloid in critically ill adults: a secondary exploratory analysis of the BaSICS clinical trial. Am J Resp Crit Care Med 2022; 205: 1419‐1428.
- 4. Zampieri FG, Machado FR, Biondi RS, et al. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the BASICS randomized clinical trial. JAMA 2021; 326: 830‐838.
- 5. Finfer S, Micaleff S, Hammond N, et al. Balanced multielectrolyte solution versus saline in critically ill adults. N Engl J Med 2022; 386: 815‐826.
- 6. Meyhoff TS, Hortrup PB, Wetterslev J, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med 2022; 386: 2459‐2470.
- 7. Lamontagne F, Richards‐Belle A, Thomas K, et al. Effect of reduced exposure to vasopressors on 90‐day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA 2020; 323: 938‐949.
- 8. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in‐hospital mortality. JAMA 2010; 303: 2165‐2171.
- 9. Mackle D, Bellomo R, Bailey M, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med 2020; 382: 989‐998.
- 10. Schjørring OL, Klitgaard TL, Perner A, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med 2021; 384: 1301‐1311.
- 11. Barrot L, Asfar P, Mauny F, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med 2020; 382: 999‐1008.
- 12. Singer M, Young PJ, Laffey JG, et al. Dangers of hyperoxia. Crit Care 2021; 25: 440.
- 13. Pandharipande PP, Girard TD, Jackson JC, et al. Long‐term cognitive impairment after critical illness. N Engl J Med 2013; 369: 1306‐1316.
- 14. Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 2018; 379: 2506‐2516.
- 15. Page VJ, Casarin A, Ely EW, et al. Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MODUS): a randomised, double‐blind, placebo‐controlled trial. Lancet Repir Med 2017; 5: 727‐737.
- 16. Reade MC, Eastwood GM, Bellomo R, et al. Effect of dexmedetomidine added to standard care on ventilator‐free time in patients with agitated delirium: a randomized clinical trial. JAMA 2016; 315: 1460‐1468.
- 17. Wibrow B, Martinez FE, Myers E, et al. Prophylactic melatonin for delirium in intensive care (Pro‐MEDIC): a randomized controlled trial. Intensive Care Med 2022; 48: 414‐425.
- 18. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46: e825‐e873.
- 19. Kentish‐Barnes N, Chevret S, Champigneulle B, et al. Effect of a condolence letter on grief symptoms among relatives of patients who died in the ICU: a randomized clinical trial. Intensive Care Med 2017; 43: 473‐484.
- 20. Showler L, Rait L, Chan M, et al. Communication with bereaved family members after death in the ICU: the CATHARTIC randomised clinical trial. Crit Care Resusc 2022; 24: 1161‐1127.
- 21. Rait LI, Yeo NY, Ali Abdelhamid Y, et al. The impact of bereavement support on psychological distress in family members: a systematic review and meta‐analysis. Crit Care Resusc 2021; 23: 225‐233.
- 22. Kentish‐Barnes N, Chevret S, Valade S, et al. A three‐step support strategy for relatives of patients dying in the intensive care unit: a cluster randomised trial. Lancet 2022; 399: 656‐664.
- 23. Writing Committee for the REMAP‐CAP Investigators. Effect of hydrocortisone on mortality and organ support in patients with severe COVID‐19. JAMA 2020; 324: 1317‐1329.
- 24. REMAP‐CAP, ACTIV‐4a, ATTACC Investigators. Therapeutic anticoagulation with heparin in critically ill patients with COVID‐19. N Engl J Med 2021; 385: 777‐789.






Open access
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
We thank Brianna Tascone and Olivia Gigli for their assistance with the production of the figure.
No relevant disclosures.