With substantial SARS-CoV-2 transmission in the community, early oral antiviral access has become a pillar of our response
Oral antiviral therapies for the treatment of mild to moderate coronavirus disease 2019 (COVID-19) are recommended in Australian guidelines to reduce the risk of serious illness, hospitalisation and mortality in people who do not require oxygen and have risk factors for progression to severe disease.1 Oral treatments should be started within five days after onset of symptoms, or as soon as practical after diagnosis in an asymptomatic individual.1 With substantial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in the community and great health system pressures and excess mortality nationwide due to COVID-19 (most of which has been experienced in 2022), early oral antiviral access has become a pillar of our response.
Eligibility and efficacy
In Australia, the two most widely available treatments for people in the community are molnupiravir (Lagevrio; MSD Australia) and combined nirmatrelvir plus ritonavir (Paxlovid; Pfizer Australia).1 These treatments were provisionally approved by the Therapeutic Goods Administration (TGA) on 18 January 2022 for treating mild to moderate COVID-19 in adults who did not require oxygen and were at increased risk of severe disease. The TGA decision was based on data from two industry-sponsored trials:2 the MOVe-OUT3 and EPIC-HR4 trials for molnupiravir and nirmatrelvir/ritonavir respectively. The treatments were initially available in February 2022 to high risk groups from the National Medical Stockpile, and then became available on the Pharmaceuticals Benefit Scheme (PBS) in March (molnupiravir) and May 2022 (nirmatrelvir/ritonavir).
The eligibility criteria were revised on 11 July 2022 to include all people aged 70 years and older, irrespective of vaccination status, and Aboriginal or Torres Strait Islander people aged 30 years and older with two or more additional risk factors (Box 1). The criteria were further updated on 1 November 2022 to include only one additional risk factor (Box 1).5 The approval and PBS listing occurred after the peak in the first Omicron wave (BA.1 and BA.2) in 2022, when there had been 3 million reported cases and the effectiveness of treatments in a vaccinated population with circulating Omicron variants was unknown.6
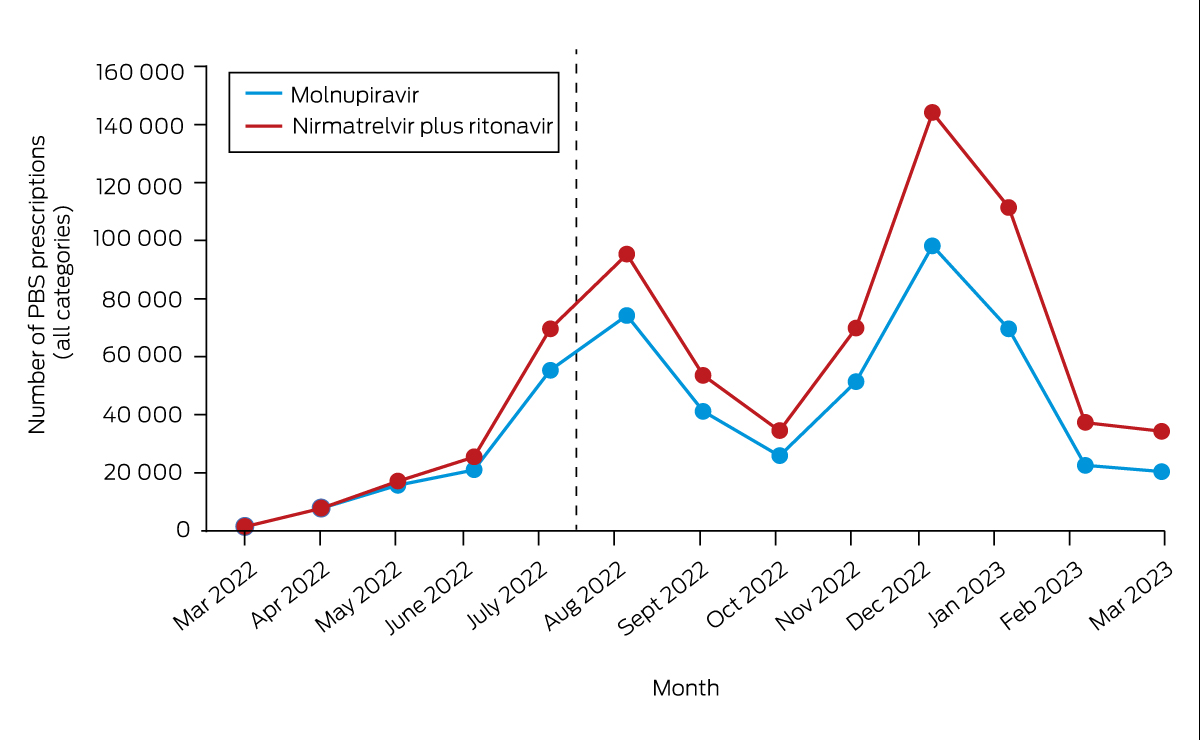
The initial clinical trials of these antivirals were done in unvaccinated populations in the pre-Omicron era.1,3,4 The nirmatrelvir/ritonavir combination had more complicated side effects and drug–drug interactions, and a contraindication profile with a greater reduction in mortality and hospitalisations than molnupiravir. The more complicated therapeutic profile of nirmatrelvir/ritonavir has likely contributed to the fact that despite being the preferred option, with 701 516 prescriptions to the end of March 2023, it was less commonly prescribed (Box 2).7 Studies on real-world effectiveness released during 2022 in waves driven by Omicron subvariants and in vaccinated populations showed that both treatments conferred benefit in reducing death and hospitalisation in the United States,4 Hong Kong8 and Victoria, Australia.9
The PANORAMIC trial (a non-industry, platform, randomised controlled trial in the United Kingdom), which randomly assigned people to usual care or molnupiravir,10 showed no impact on hospitalisation or death in a vaccinated population during the Omicron era. There was a quicker time to recovery reported, which is a subjective measure open to bias. The PANORAMIC trial results have led Australia's National Clinical Evidence Taskforce recommend against “the routine use of molnupiravir”.11 Health departments in Australia have continued to recommend molnupiravir only if the nirmatrelvir/ritonavir combination cannot be prescribed, noting that the risk of hospitalisation and death in both arms of the PANORAMIC trial was substantially lower than that observed in real-world data in the high risk populations for whom antivirals are recommended in Australia.9
Prolonged illness following COVID-19 (often referred to as “long COVID”) is predicted to cause a considerable burden on affected individuals and the health system, with an incidence of 5–10%.12 Severe disease is a risk factor for prolonged persistent symptoms; therefore, it is plausible, but not yet proven, that antiviral therapy might help reduce long term sequelae. The majority of patients in the most affected group (35–49-year-olds) are not currently eligible for treatment in Australia.13,14 Trials underway, including the PANORAMIC trial, will evaluate the effect of antiviral treatment on the incidence of persistent symptoms.10
Equity of access
During the pandemic, successive waves of COVID-19 have disproportionately affected people in more vulnerable groups in society who have experienced higher rates of infection, hospitalisation and death.3,15 In the United States, disadvantage has been compounded, with people living in areas of high social vulnerability being less likely to access treatments for COVID-19 than those from areas of low social vulnerability.16 Studies in the UK report that the disadvantaged and ethnic minority communities are also at higher risk of long COVID.14
Australia experienced a reduction in overall excess mortality in the first two years of the pandemic due to public health measures, including lockdowns.17 There was a disproportionate burden of COVID-19-related mortality for older Australians, particularly in residential aged care facilities; people from lower socio-economic backgrounds; Aboriginal and Torres Strait Islander people; people born overseas; and those living with a disability.17 Environmental and socio-economic factors, such as large household size, crowding, and work settings, contributed to an increased risk of infection in these groups. During the Omicron wave to July 2022, the rate of severe disease (defined as intensive care unit admission or death) in Aboriginal and Torres Strait Islander people was 1.4 times higher than in non-Aboriginal and Torres Strait Islander people, and through the pandemic to 30 April 2022, people born in North Africa and the Middle East were seven times more likely to die of COVID-19 than people born in Australia.17 The deaths attributed to COVID-19 underestimate the true burden of the pandemic in terms of lives lost due to under-reporting of positive rapid antigen tests (RATs), people becoming sick or dying before testing, and late disease effects, particularly cardiac events causing COVID-19-related mortality that may not be recorded as related to COVID-19.18
Before 2022, diagnosis was done via laboratory polymerase chain reaction (PCR) tests, with mandatory notification, and there were programs to provide systematic care to notified cases.19 With the explosion in case numbers and the rollout of RATs, the burden of reporting and negotiating timely care is now on the individual. The Omicron waves have come at a time when there is a crisis in general practice due to workforce shortages and a failure of indexation of Medicare items to the consumer price index, complicated by pandemic-related burnout across the health system.20 There is an increased demand for appointments due to delayed care and an increasing number of general practitioners abandoning bulk-billing.20
In response to early issues of limited supply of antivirals, stock was placed in residential aged-care facilities and Aboriginal Community Controlled Health Services, and telehealth was extended to assist with access to GP appointments.21 General practice respiratory clinics, funded by federal, state and territory governments, were able to provide an access point for COVID-19 antivirals.22 GPs were encouraged to assess vulnerable patients for an antiviral treatment and to have planning discussions before SARS-CoV-2 infection. Overseas examples of attempts to ensure equity include the establishment of test to treat facilities in the United States to increase access and reduce deaths in disproportionally affected populations. In New Zealand, prescribing was extended to include pharmacists to reduce barriers to accessing doctor or nurse appointments before accessing treatment,23 which has ignited the debate about the role of pharmacist prescribing in Australia.
In September 2022, data from the Victorian Department of Health were analysed to understand equity in antiviral rollout. Data from the PBS and the Transmission and Response Epidemiology Victoria (TREVI) database — a COVID-19 surveillance database from the Victorian Department of Health — were linked, using name, surname, and date of birth as linkage key and then de-identified for analysis. Analysis on time to treatment was descriptive. Index of Relative Socio-economic Disadvantage (IRSD) scores at the postcode level area were obtained from the Australian Bureau of Statistics. Treatment uptake was compared between the lower and upper IRSD quartiles; the odds of treatment given were calculated for each IRSD group. The odds ratio and the maximum likelihood confidence intervals were calculated. The proportion of reported COVID-19 cases in people aged 70 years and older who had a linked treatment with antivirals was 64.4%. Treatment coverage was estimated to be higher, as nearly 40% of treatments in people aged over 70 years were not linked to a reported case. For time to treatment, 95% of oral antivirals were supplied within three days of diagnosis. This metric was consistent across IRSD deciles, except for the top IRSD decile postcodes, where 95% of cases received treatment within two days from diagnosis. There were no differences in median time to treatment when comparing Aboriginal and Torres Strait Islander people and non-Aboriginal and Torres Strait Islander people or those living in metropolitan and rural areas. Reported cases from the lowest IRSD quartile were 15% (95% CI, 13–17%) less likely to receive oral antivirals compared with those in the top IRSD quartile. A limitation of analysis based on linking reported cases is the potential bias due to selection of subjects with a higher level of access to care and incentives or knowledge to test and register their infection. These people may also be more likely to access antiviral treatments.
The new population eligibility criteria for COVID-19 oral antivirals are simplified. Supply has improved and prescribing has increased,7 so equity must now be our top priority. Equity depends on continuing to address the structural inequalities within our health system that create barriers to people accessing primary health services and tailoring responses to communities.
Improved data reporting through linkage of large datasets can track how treatments are delivered across the health system by demographic factors. COVID-19 mortality and morbidity data should include information on vaccination status, and both eligibility and receipt of COVID-19 oral antivirals. Real-time national analysis of the current rollout can ensure policy is informed by evidence and enable investment in health systems to improve access where gaps are identified. These approaches have been instrumental in addressing equity of vaccination uptake in the Australian population.
Equitable delivery of health care is a perpetual challenge. Better investment in general practice, community health and Aboriginal Controlled Community Health Services will assist to uphold equity in the response to COVID-19. If Australia reverts completely to a “business as usual model”, relying on stretched primary health care services with no additional resourcing (including addressing Medicare indexation as a matter of urgency), we will fail to reach the people likely to benefit from treatment. For millennia, pandemics have disproportionately affected those who are disadvantaged. We have an opportunity to learn from this pandemic and ensure antivirals for COVID-19 are at the forefront of a better health response and are used to reduce existing disparities in hospitalisation and death — not to further entrench them.
Box 1 – Eligibility criteria changes under the Pharmaceutical Benefits Scheme (PBS)
|
Population |
Eligibility criteria at initial PBS listing |
Expanded criteria from 11 July 2022 |
Expanded criteria from 1 November 2022 |
Expanded criteria from 1 January 2023 |
Expanded criteria (for Paxlovid* only) from 1 April 2023 |
||||||||||
|
|
|||||||||||||||
|
Aboriginal or Torres Strait Islander people |
= 50 years and at high risk† |
= 30 years with two additional risk factors |
= 30 years with one additional risk factors |
No change |
No change |
||||||||||
|
Moderately to severely immunocompromised patients‡ |
= 18 years |
No change |
|
Addition to include CD20 monoclonal antibodies in past 12 months |
No change |
||||||||||
|
Age and risk |
= 65 years and at high risk |
= 50 years with two additional risk factors |
No change |
= 18 years and history of being previously hospitalised for COVID-19 |
60–69 years with mild to moderate disease and one additional risk factor |
||||||||||
|
Age only |
na |
= 70 years |
No change |
No change |
No change |
||||||||||
|
|
|||||||||||||||
|
COVID-19 = coronavirus disease 2019; na = not applicable. * Pfizer Australia. † High risk criteria: residential aged care resident; disability with multiple comorbid conditions and/or frailty; neurological conditions; respiratory compromise, including chronic obstructive pulmonary disease, moderate or severe asthma, bronchiectasis; heart failure; coronary artery disease; cardiomyopathies; obesity (body mass index > 30 kg/m2); type 1 or type 2 diabetes requiring medication; renal impairment (estimated glomerular filtration rate < 60 mL/min); cirrhosis; or living in a geographically remote area. ‡ Immunosuppression: chemotherapy or whole-body radiotherapy; high dose corticosteroids (prednisone = 20 mg per day, or equivalent); biological agents and treatments that deplete or inhibit B cell or T cell function; selected conventional synthetic disease-modifying antirheumatic drugs; alkylating agents; any significantly immunocompromising conditions where, in the past 12 months, the patient has received rituximab; high risk conditions, including Down syndrome, cerebral palsy, congenital heart disease, thalassaemia, sickle cell disease and other haemoglobinopathies; people with disability with multiple comorbid conditions and/or frailty. |
|||||||||||||||
Provenance: Commissioned; externally peer reviewed.
- 1. National Clinical Evidence Taskforce, COVID‐19. Caring for people with COVID‐19: living guidelines. National Clinical Evidence Taskforce, 2022. https://clinicalevidence.net.au/covid‐19/#living‐guidelines (viewed Mar 2023).
- 2. Therapeutic Goods Administration. COVID‐19 treatments: provisional registrations 2022 [updated 15 Aug 2022]. TGA, 2022. https://www.tga.gov.au/covid‐19‐treatments‐provisional‐registrations (viewed Mar 2023).
- 3. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID‐19 in nonhospitalized patients. N Engl J Med 2022; 386: 509‐520.
- 4. Ganatra S, Dani SS, Ahmad J, et al. Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019. Clin Infect Dis 2022; 76: 563‐572.
- 5. Australian Government Department of Health and Aged Care. Updated eligibility for oral COVID‐19 treatments. Canberra: Commonwealth of Australia, 2022. https://www.health.gov.au/health‐alerts/covid‐19/treatments/eligibility#eligibility‐for‐oral‐covid19‐treatments (viewed Mar 2023).
- 6. Australian Government Department of Health and Aged Care. Coronavirus (COVID‐19) case numbers and statistics. Canberra: Commonwealth of Australia, 2022. https://www.health.gov.au/health‐alerts/covid‐19/case‐numbers‐and‐statistics (viewed Mar 2023).
- 7. Australian Government, Services Australia. Pharmaceutical Benefits Schedule item reports 2022. Canberra: Commonwealth of Australia, 2022. http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp (viewed May 2023).
- 8. Wong CKH, Au ICH, Lau KTK, et al. Real‐world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID‐19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis 2022; 22: 1681‐1693.
- 9. Cowie B, Sutton B, Van Heer C, Majumdar S. Paxlovid is Australia's first‐line COVID antiviral but Lagevrio also prevents severe disease in over‐70s. The Conversation 2022; 29 Nov. https://theconversation.com/paxlovid‐is‐australias‐first‐line‐covid‐antiviral‐but‐lagevrio‐also‐prevents‐severe‐disease‐in‐over‐70s‐195349 (viewed Mar 2023).
- 10. Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID‐19 at increased risk of adverse outcomes (PANORAMIC): an open‐label, platform‐adaptive randomised controlled trial. Lancet 2023; 401: 281‐293.
- 11. National Clinical Evidence Taskforce. Communique #76; 02 December 2022. https://clinicalevidence.net.au/news/communique‐76/ (viewed Mar 2023).
- 12. Australian Institute of Health and Welfare. Long COVID in Australia — a review of the literature [Cat. No. PHE 318]. AIHW, 2022. https://www.aihw.gov.au/reports/covid‐19/long‐covid‐in‐australia‐a‐review‐of‐the‐literature/summary (viewed Mar 2023).
- 13. Heidi Ledford. Can drugs reduce the risk of Long COVID? What scientists know so far. Nature 2022; 604: 20‐21.
- 14. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non‐hospitalized adults. Nat Med 2022; 28: 1706‐1714.
- 15. Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID‐19‐related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med 2021; 174: 362‐373.
- 16. Gold JAW, Kelleher J, Magid J, et al. Dispensing of oral antiviral drugs for treatment of COVID‐19 by zip code — level social vulnerability — United States, December 23, 2021 – May 21, 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 825‐829.
- 17. Australian Institute of Health and Welfare. Australia's health 2022: data insights [Cat. No. AUS 240]. AIHW, 2022. https://www.aihw.gov.au/reports/australias‐health/australias‐health‐2022‐data‐insights/about (viewed Mar 2023).
- 18. Xie Y, Xu E, Bowe B, Al‐Aly Z. Long‐term cardiovascular outcomes of COVID‐19. Nat Med 2022; 28: 583‐590.
- 19. Lim SM, Allard NL, Devereux J, et al. The COVID Positive Pathway: a collaboration between public health agencies, primary care, and metropolitan hospitals in Melbourne. Med J Aust 2022; 216: 413‐419. https://www.mja.com.au/journal/2022/216/8/covid‐positive‐pathway‐collaboration‐between‐public‐health‐agencies‐primary‐care
- 20. Royal Australian College of General Practitioners. General practice: Health of the Nation 2022. RACGP, 2022. https://www.racgp.org.au/getmedia/80c8bdc9‐8886‐4055‐8a8d‐ea793b088e5a/Health‐of‐the‐Nation.pdf.aspx (viewed Mar 2023).
- 21. Australian Government Department of Health and Aged Care. MBS telehealth services from 1 July 2022. Canberra: Commonwealth of Australia, 2022. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Factsheet‐telehealth‐1July22 (viewed Nov 2022).
- 22. Davis S, Roberts L, Desborough J, et al. Integrating general practice into the Australian COVID‐19 response: a description of the General Practitioner Respiratory Clinic Program in Australia. Ann Fam Med 2022; 20: 273‐276.
- 23. New Zealand Government, Ministry of Health. Medicines to treat COVID‐19. New Zealand Government, 2022. https://www.health.govt.nz/covid‐19‐novel‐coronavirus/covid‐19‐health‐advice‐public/advice‐people‐covid‐19/covid‐19‐medicines (viewed Mar 2023).






Open access:
Open access publishing facilitated by The University of Melbourne, as part of the Wiley – The University of Melbourne agreement via the Council of Australian University Librarians.
No relevant disclosures.