Future threats require an integrated approach to the health of humans, animals and the environment
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has clearly demonstrated our global vulnerability to emerging infectious diseases. Zoonoses — diseases that transmit from vertebrate animals to humans — are twice as likely to be implicated as emerging diseases than non‐zoonoses.1 Such diseases have been increasingly linked to wildlife, which are a source of infection for humans and domestic animals,2 with viral spillover driven by human‐induced changes in land use, agricultural intensification, and wildlife exploitation, among other things.3 Sadly, warnings from experts about the dangers of unsustainable development and its impact on natural systems remained largely unheeded by politicians and policymakers.3
Meanwhile, SARS‐CoV‐2 continues to produce surprises. The virus was recently confirmed to be spreading in white‐tailed deer in North America, and there are concerns that they may become a reservoir.4 Closer to home, the ability of cats to become infected with SARS‐CoV‐2 from their owners left experts grappling with the question of whether they could pass the virus on to humans.5 With 27% of Australian households owning a cat,6 such transmission would have amplified the burden of coronavirus disease 2019 (COVID‐19) on the health system and would have presented considerable ethical and welfare dilemmas for veterinarians. A response would have required coordinated action by human and animal health professionals, which is unprecedented for a disease affecting companion animals and is largely unsupported by current systems. Fortunately, this scenario did not eventuate; there has only been one confirmed instance of cat‐to‐human infection (in a veterinarian),7 but a future emerging disease may behave differently. Indeed, similar discussions are now occurring in relation to monkeypox and the potential for pet rodents to become reservoirs.8
Globally, the emergence of new diseases has increased over recent decades,9 with homegrown examples being the Hendra virus and the Australian bat lyssavirus.10 In 2022, the detection of Japanese encephalitis — an arthropod‐borne viral zoonoses that is thought to have entered Australia through movement of infected mosquitoes or migratory waterbirds11 — demonstrates how easy it is for diseases to enter the country despite our island status. Australia is the only country in the Organisation for Economic Co‐operation and Development (OECD) that does not have a national Centre for Disease Control (CDC). Under current structures, the national coordination and leadership for prevention and control of communicable diseases, including zoonoses, are led by the Communicable Diseases Network Australia. Formal representation of animal health professionals within this structure is limited to one veterinarian.12 The management of zoonotic diseases outbreaks depends on established working relationships and protocols between federal and state or territory human and animal health departments, the strength of which varies across jurisdictions.13
Improving Australia’s capacity to prevent, detect, respond to, and recover from zoonotic outbreaks and other health emergencies requires a re‐think of this approach. Indeed, the unprecedented and complex issues presented by the COVID‐19 pandemic highlighted the need for greater multisectoral engagement in both planning and implementation of pandemic preparedness in Australia, even when there is limited zoonotic transmission.14 Worldwide, there is growing recognition that protection of human health requires a collaborative approach that can more nimbly tackle problems at the interface of human, animal and environmental health. This way of working is realised by the concept of One Health, an approach that was recently endorsed by G20 Health Ministers15 and the Quadripartite, comprised of the Food and Agriculture Organization of the United Nations (FAO), the World Organisation for Animal Health (WOAH, formerly OIE), the United Nations Environment Programme (UNEP) and the World Health Organization (WHO).16 The new definition adopted by the Quadripartite (Box 1) makes clear that, beyond issues such as zoonoses, antimicrobial resistance and food safety, One Health is foremost about contributing to more sustainable development of the planet.
We believe that the operationalisation of One Health in Australia — that is, improving the way that sectors work together to tackle threats to health and ecosystems — can be partly realised through innovations to our health system. Key aspects of our vision of this One Health system, which extends WHO’s health system building blocks framework18 to consider human, animal and environmental health, are described below and illustrated in Box 2.
First, although it is necessary to maintain stand‐alone human, animal and environmental health governance structures given their different remits, there is a clear need to establish a One Health governance mechanism to provide leadership and foster collaboration, coordination and communication between sectors and the community. This could be achieved by embedding a One Health coordination mechanism in a newly formed Australian CDC, facilitating cooperative and equitable engagement across sectors. This would allow actors to transcend traditional boundaries and bring diverse expertise, resources and perspectives in order to address shared health threats.19 In addition, benefits gained by a platform for One Health coordination would facilitate integrated engagement in areas beyond the common foci of zoonoses, antimicrobial resistance and food safety, to include non‐communicable diseases and the impacts of climate change on health.
Second, sharing of health intelligence information across sectors would enable timely risk assessment and early response to zoonoses20 and to other environmental hazards affecting humans, animals, and ecosystem integrity. This would require compatible information technology systems that facilitate joint analysis and dissemination while maintaining data confidentiality. It would also be aided by greater sharing of laboratory infrastructure between human and animal health services, particularly in rural and remote areas, which would benefit from increased access to diagnostic facilities. This type of arrangement is not without precedent in Australia. Animal health laboratories added substantial surge capacity for SARS‐CoV‐2 testing during the COVID‐19 pandemic,14 and the Australian Centre for Disease Preparedness — a federal animal health laboratory in Geelong, Victoria — already provides diagnostic and research capability for dangerous zoonotic pathogens.
Third, clinical practitioners require resources and training to respond to zoonoses using collaborative approaches. Optimising outcomes for zoonoses requires input from both human and animal health practitioners who have different but complimentary roles. Even though the primary role of doctors is to manage disease in their human patients, veterinarians are trained in recognising and managing risks posed by zoonoses, as well as implementing treatment in animal patients where indicated. Inclusion of One Health in clinical training and continuing professional education would build workforce capacity of frontline service providers, strengthening knowledge and skills in relevant areas and also facilitating mutual understanding of the complementary skill sets of each profession.21
Fourth, integrated management of zoonoses would provide more efficient and cost‐effective delivery of health services. Whereas a general practitioner may refer a patient to an allied health professional for further management, there is currently no formal mechanism for referral of a patient at high risk for toxoplasmosis to a veterinarian for advice on managing cat‐related risks, for example. Similarly, a veterinarian who diagnoses Brucella suis infection in a hunting dog can only informally advise owners to consult a general practitioner about their personal risk of infection when hunting feral pigs. Formal incorporation of veterinarians into the health system as allied health professionals who have specialist training in zoonoses would improve both continuity of care and health outcomes through a more holistic approach to management of both human and animal patients. Enacting this clearly requires legislative change, and it is notable that the practice of cross‐professional referral in the case of zoonoses management has been favourably received in surveys with the public22 and general practitioners23 in Australia. Beyond zoonoses, referral could provide additional benefits in areas such as animal‐assisted therapies and management of work, health and safety issues in animal industries.
Fifth, although collaborative approaches to research and development and regulation of medical products are not new (emulated by the fields of “One Medicine” and “comparative pathology”), a One Health system would require shared regulatory responsibility for medications used in humans, animals and horticultural industries, as well as management of the impact of pharmaceutical pollution on ecosystem health.24 Of particular concern, environmental exposure of microbes to antimicrobials facilitates selection for antimicrobial resistance. This needs to be managed alongside antimicrobial stewardship programs in human and animal health to ensure continued treatment success.
Sixth, in a One Health system, investigation and response to zoonoses could be jointly financed by human and animal health sectors, proportionate to the impact on each sector. Cost sharing between government and industry is already a feature of Australia’s response to emergency animal diseases and plant pests.25 These arrangements could be expanded to consider a scenario where the primary beneficiary of zoonoses control is the human health sector, through curbing the burden of human disease. Notably, unlike in human medicine, where Australians have access to highly subsidised care through Medicare, costs of veterinary interventions are largely born by animal owners, creating barriers to laboratory investigation.26 Under a One Health system, costs incurred when ruling out a zoonotic disease or performing culture and sensitivity tests to inform antibiotic prescription in an animal patient could be considered an eligible cost under an expanded Medicare scheme, due to the implications for human health.
The need for a national CDC is the subject of current debate in Australia. Its establishment is supported by the Labor government, the Australian Medical Association, the Public Health Association of Australia, the Australasian Society for Infectious Diseases, the Australasian College for Infection Prevention and Control, and the Australian Society for Antimicrobials. Further, the Australian Veterinary Association has called for the establishment of a One Health framework for disease prevention and control within Australia in their national election platform.27 Although CDCs around the world, including in the United States, Europe and Africa, have embraced One Health, this has not yet been a topic of substantive discussion in Australia. We implore policymakers to seize this opportunity to create a truly integrated centre that can tackle future health threats through fostering multisectoral, One Health approaches in Australia.
Box 1 – Definition of One Health, as developed by the One Health High‐Level Expert Panel and adopted by the Quadripartite (FAO, WOAH, UNEP, WHO)
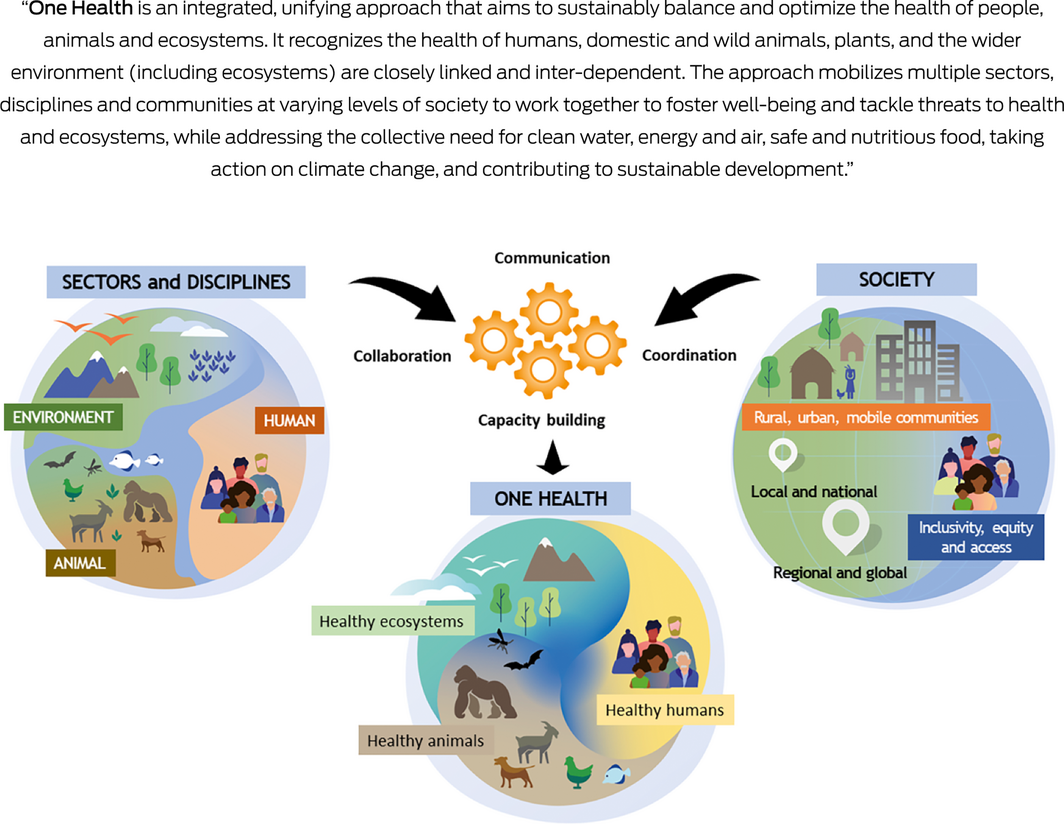
FAO = Food and Agriculture Organization of the United Nations; UNEP = United Nations Environment Programme; WOAH = World Organisation for Animal Health; WHO = World Health Organization. Source: Figure reproduced from One Health High‐Level Expert Panel (OHHLEP) et al.17
Box 2 – A vision of a One Health system for Australia
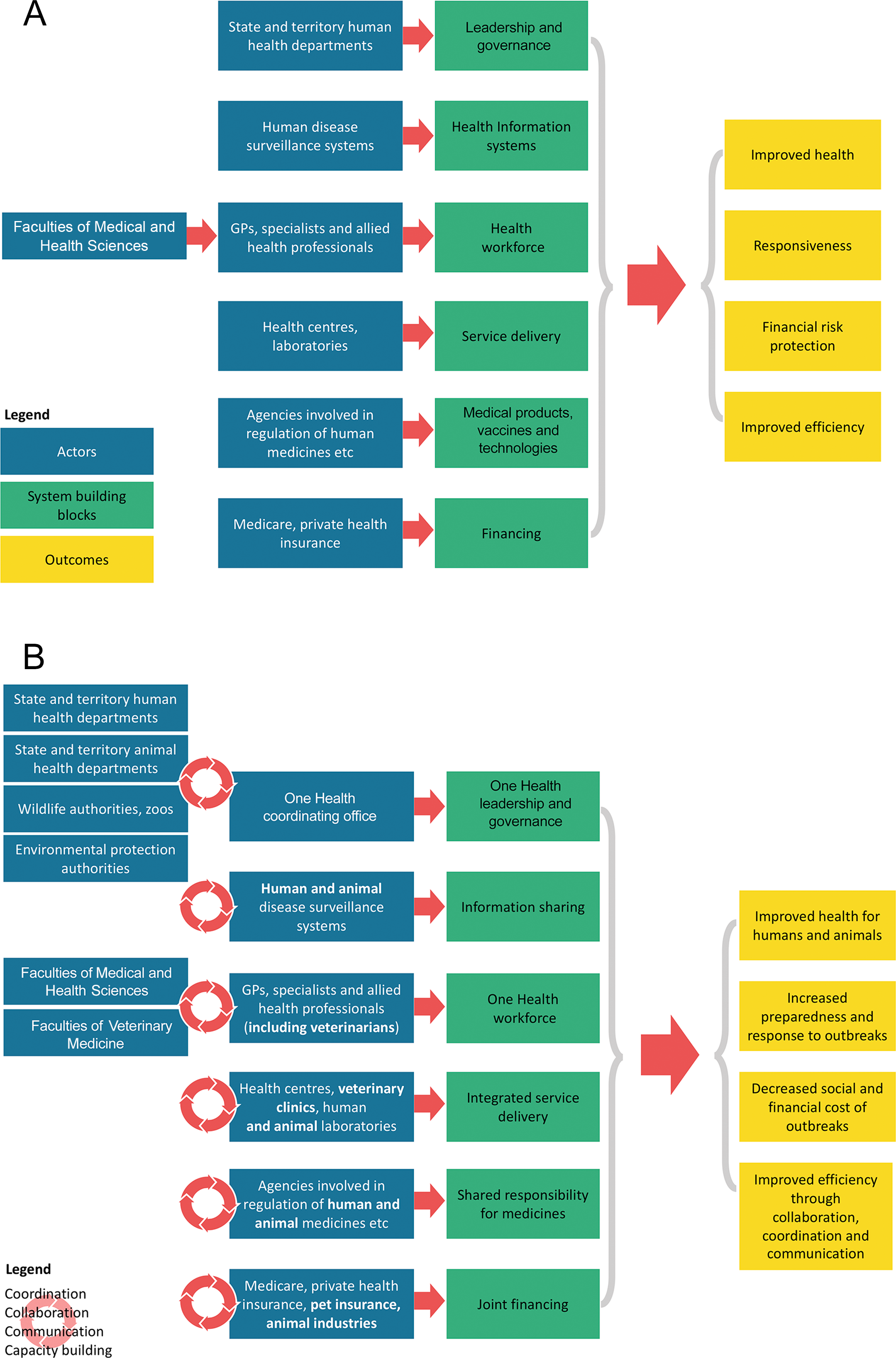
GPs = general practitioners. Panel A (conventional view of the health system) depicts the health system as the sum of six building blocks (green). Each building block requires contributions from different actors (blue) who work together to produce the desired outcomes (yellow). Panel B (One Health system) depicts the health system as the sum of contributions by all actors towards human health, including animal health agencies, industries and professionals, as well as environmental protection agencies. Enhanced coordination, collaboration, communication, and capacity building between these actors produce improved health for all species, resulting in more effective prevention and increased preparedness and response to zoonotic outbreaks, mitigating the health, social and financial costs to all sectors.
Provenance: Commissioned; externally peer reviewed.
- 1. Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 2001; 356: 983‐989.
- 2. Cox‐Witton K, Reiss A, Woods R, et al. Emerging infectious diseases in free‐ranging wildlife — Australian zoo based wildlife hospitals contribute to national surveillance. PLoS One 2014; 9: e95127.
- 3. Gruetzmacher K, Karesh WB, Amuasi JH, et al. The Berlin principles on One Health — bridging global health and conservation. Sci Total Environ 2021; 764: 142919.
- 4. Mallapaty S. COVID is spreading in deer. What does that mean for the pandemic? Nature 2022; 604: 612‐615.
- 5. Banerjee A, Mossman K, Baker ML. Zooanthroponotic potential of SARS‐CoV‐2 and implications of reintroduction into human populations. Cell Host Microbe 2021; 29: 160‐164.
- 6. Animal Medicines Australia. Pets in Australia: a national survey of pets and people. Animal Medicines Australia, 2019. https://animalmedicinesaustralia.org.au/report/pets‐in‐australia‐a‐national‐survey‐of‐pets‐and‐people/ (viewed Sept 2022).
- 7. Sila T, Sunghan J, Laochareonsuk W, et al. Suspected cat‐to‐human transmission of SARS‐CoV‐2, Thailand, July–September 2021. Emerg Infect Dis 2022; 28: 1485‐1488.
- 8. UK Health Security Agency. Qualitative assessment of the risk to the UK human population of monkeypox infection in a canine, feline, mustelid, lagomorph or rodent UK pet [updated 27 May 2022]. UKHSA, 2022. https://www.gov.uk/government/publications/hairs‐risk‐assessment‐monkeypox/qualitative‐assessment‐of‐the‐risk‐to‐the‐uk‐human‐population‐of‐monkeypox‐infection‐in‐a‐canine‐feline‐mustelid‐lagomorph‐or‐rodent‐uk‐pet (viewed Sept 2022).
- 9. Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature 2008; 451: 990‐993.
- 10. NSW Government, Department of Health. Rabies and Australian bat lyssavirus infection fact sheet [updated 26 July 2019]. https://www.health.nsw.gov.au/Infectious/factsheets/Pages/rabies‐australian‐bat‐lyssavirus‐infection.aspx (viewed Sept 2022).
- 11. Department of Agriculture, Fisheries and Forestry. National pest and disease outbreaks 2022 https://www.outbreak.gov.au/ (viewed Sept 2022).
- 12. Australian Governments, Department of Health and Aged Care. CDNA members, 2020. https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda‐cdna‐cdmembrs.htm (viewed Sept 2022).
- 13. Adamson S, Marich A, Roth I. One Health in NSW: coordination of human and animal health sector management of zoonoses of public health significance. NSW Public Health Bull 2011; 22: 105‐112.
- 14. Steele SG, Toribio JALML, Mor SM. Global health security must embrace a One Health approach: Contributions and experiences of veterinarians during the COVID‐19 response in Australia. One Health 2021; 13: 100314.
- 15. Global Health Summit. The Rome Declaration. Rome: G20; 2021. https://global‐health‐summit.europa.eu/rome‐declaration_en (viewed Sept 2022).
- 16. World Health Organization. Quadripartite Memorandum of Understanding (MoU) signed for a new era of One Health collaboration 2022 [updated 29 Apr 2022]. https://www.who.int/news/item/29‐04‐2022‐quadripartite‐memorandum‐of‐understanding‐(mou)‐signed‐for‐a‐new‐era‐of‐one‐health‐collaboration (viewed Sept 2022).
- 17. One Health High‐Level Expert Panel (OHHLEP); Adisasmito WB, Almuhairi S, Behravesh CB, et al. One Health: a new definition for a sustainable and healthy future. PLoS Pathog 2022; 18: e1010537.
- 18. World Health Organization. Everybody’s business — strengthening health systems to improve health outcomes: WHO’s framework for action. Geneva: WHO; 2007. https://apps.who.int/iris/handle/10665/43918 (viewed Sept 2022).
- 19. Choi BCK, Pak AWP. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research, services, education and policy: 1. Definitions, objectives, and evidence of effectiveness. Clin Invest Med 2006; 29: 351‐364.
- 20. Food and Agriculture Organization of the United Nations, United Nations Environment Programme, World Health Organization, World Organisation for Animal Health. Enhancing One Health intelligence to strengthen global health security — the One Health Intelligence Scoping Study. https://www.fao.org/publications/card/en/c/CC0003EN/ (viewed Sept 2022).
- 21. Rabinowitz P, Conti L. Human–animal medicine: clinical approaches to zoonoses, toxicants and other shared health risks. Missouri: Saunders Elsevier, 2010.
- 22. Speare R, Mendez D, Judd J, et al. Willingness to consult a veterinarian on physician’s advice for zoonotic diseases: a formal role for veterinarians in medicine? PLoS One 2015; 10: e0131406.
- 23. Steele SG, Booy R, Manocha R, et al. Towards One Health clinical management of zoonoses: a parallel survey of Australian general medical practitioners and veterinarians. Zoonoses Public Health 2021; 68: 88‐102.
- 24. Wilkinson JL, Boxall ABA, Kolpin DW, et al. Pharmaceutical pollution of the world’s rivers. Proc Nat Acad Sci USA 2022; 119: e2113947119.
- 25. Animal Health Australia. Animal health in Australia system report, 1st ed. Canberra: Animal Health Australia, 2021. https://animalhealthaustralia.com.au//wp‐content/uploads/dlm_uploads/2021/04/AHAH2001_Dan‐AHiA‐2020‐Systems‐Report_FA2_Digital‐min.pdf (viewed Sept 2022).
- 26. Steele SG, Mor SM, Toribio JALML. “It’s our job”: constraints to investigation of atypical disease events — opinions of Australian veterinarians. Zoonoses Public Health 2021; 68: 493‐502.
- 27. Australian Veterinary Association. AVA National Election Platform. https://www.ava.com.au/siteassets/advocacy/ava‐national‐election‐platform‐2022.pdf (viewed Sept 2022).






Open access
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
We thank Professor Robert Booy for his assistance and support of our research.
No relevant disclosures.