The known: Anaemia, common in patients undergoing major surgery, has a negative impact on patient outcomes. The Australian National Blood Authority has guidelines for appropriately identifying and managing peri‐operative anaemia.
The new: Peri‐operative investigation and management of anaemia in patients undergoing major abdominal surgery in Australia and New Zealand is variable. Not adhering to pre‐operative assessment guidelines was associated with poorer patient outcomes. In particular, the 30‐day re‐admission rate was higher for the 59% of patients discharged from hospital with anaemia.
The implications: The management of peri‐operative anaemia after major abdominal surgery should be standardised to improve patient outcomes.
About one‐third of patients who present for major surgery have anaemia, and as many as three‐quarters have it when discharged from hospital.1 Peri‐operative anaemia is associated with higher post‐operative complication rates, longer hospital stay, poorer quality of life, and delayed recovery.1,2
In their patient blood management guidelines, the Australian National Blood Authority (ANBA) and the Association of Anaesthetists of Great Britain and Ireland (AAGBI) recommend that anaemia be identified, evaluated, and managed prior to surgery,3,4 that tranexamic acid be administered during the procedure if blood loss sufficient to cause anaemia is anticipated,3 and that a restrictive post‐operative blood transfusion strategy be applied,3,4 haemoglobin routinely assessed, and early oral iron administration be avoided after surgery.4
The treatment of anaemia with intravenous iron has increased in Australia during the past decade.5 However, a recent multicentre trial in the United Kingdom (Preoperative intravenous iron to treat anaemia before major abdominal surgery, PREVENTT) found that it provided no benefit to patients with pre‐operative anaemia undergoing major abdominal surgery, although a possible association between correction of post‐operative anaemia and lower number of hospital re‐admissions was noted.6
The POST‐operative Variability in anaemia treatmenT and Transfusion (POSTVenTT) study examined the management of peri‐operative anaemia in Australia and New Zealand (Aotearoa), audited adherence to anaemia management guidelines after major abdominal surgery, and assessed their association with patient outcomes.
Methods
The POSTVenTT study protocol has been published,7 and the trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12621001517864; 8 November 2021). We report the trial in concordance with the Strengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.8
POSTVenTT was a prospective, multicentre, observational cohort study undertaken by student‐ and trainee‐led collaborative networks in Australia and New Zealand, supported by the Royal Australasian College of Surgeons’ Clinical Trials Network Australia and New Zealand and the Western Australia Clinical Trials Unit. All hospitals performing abdominal surgery were eligible and invited to participate. Data were collected by teams of three to five medical students and trainees during two 2‐week periods in July 2021, followed by prospective follow‐up of patients for 30 days after discharge from hospital, to 12 September 2021. A student or trainee leader was responsible for organising local teams and governance, supported by a consultant. All collaborators completed training in good clinical practice prior to study commencement. Data were collected in case report forms and subsequently transferred to the secure REDCap database hosted by the University of Western Australia data centre.
Data collection
Data for all consecutive adult patients (18 years or older) who underwent major emergency or elective abdominal surgery at the 56 participating hospitals were included in our analysis. Major abdominal surgery was defined as any operation with an incision into the abdominal cavity (open, laparoscopic, or robotic surgery) and anticipated duration of more than one hour. Further details on eligible surgical procedures and data items collected are provided in the published study protocol7 and in the Supporting Information, supplementary methods.
Definitions and outcomes
Anaemia was defined by World Health Organization sex‐specific cut‐off values (haemoglobin < 130 g/L for men, < 120 g/L for non‐pregnant women).9 The primary outcomes were the proportions of patients managed according to the five audit standards for pre‐, intra‐, and post‐operative patient blood management, based on ANBA3 and AAGBI4 consensus guidelines (Box 1). Secondary outcomes were intra‐ and post‐operative packed red blood cell (pRBC) transfusion, post‐operative complications (Clavien–Dindo classification),10 clinical frailty score (CSHA Clinical Frailty Scale),11 length of hospital stay, hospital re‐admission within 30 days of discharge, and the prevalence of anaemia prior to surgery, on days 1 to 3 after surgery (nadir haemoglobin), at the final haemoglobin measurement before hospital discharge, and 30 days after discharge.
Statistical analysis
All analyses (Supporting Information, supplementary methods) were undertaken in R 3.6.1 (R Foundation for Statistical Computing). Categorical data are reported as numbers and proportions, and the statistical significance of differences was assessed in χ2 tests. Continuous data are reported as means with standard deviations (SDs) or medians with interquartile ranges (IQRs), and the statistical significance of differences was assessed in independent samples t tests or Kruskal–Wallis tests. Length of stay was analysed as time‐to‐event in log‐rank tests, with censoring for patients not discharged by end of study follow‐up. Variation between hospitals was visually assessed in unadjusted funnel plots with 95% and 99% confidence intervals.
We assessed the relationships between audit adherence and patient outcomes in multivariate analyses (binary logistic or Cox regression models), and report adjusted odds ratios (aORs) or hazard ratios (HRs) with 95% confidence intervals (CIs). Potential explanatory variables (age, sex, ethnic background, surgical specialty, surgical urgency) were included in these models a priori because of their recognised influence on surgical outcomes. Missing data were excluded in a pairwise manner.
We pre‐specified that data be included only from hospitals for which data completeness exceeded 95%; this threshold was reached for all participating centres. A post hoc sensitivity analysis excluded patients who underwent cholecystectomy. Although defined as major surgery, cholecystectomy is often performed as a day procedure, and the risks of bleeding and anaemia are generally lower than for other operations.
Ethics approval
Our study was approved by the South Metropolitan Health Service (Western Australia) Health Research Ethics Committee (EC00265), the Western Australia Research Governance System (RCG 4477), and the Auckland Health Research Ethics Committee (AH21859). Each committee waived the requirement for individual patient consent for this audit of routine clinical data.
Results
Data were available for 2730 eligible patients from 56 hospitals (median, 49.5 [IQR, 21–76] patients per hospital); their mean age was 56.7 years (SD, 17.3 years), 1558 were women (57.1%). The most frequent operation types were hepatopancreatobiliary (1224 patients, 44.8%), colorectal (760, 27.8%), and upper gastrointestinal surgery (436, 16.0%); 687 patients underwent urgent or immediate surgery (25.2%). A total of 671 patients experienced in‐hospital complications (24.6%), including 186 (6.8%) with major complications (Clavien–Dindo grade III or higher) (Box 2).
By the close of the study, 2635 patients had been discharged (96.5%), after a median hospital stay of three days (IQR, 1–7 days); 33 people had died in hospital (1.2%). Follow‐up 30 days after discharge was completed for 2585 patients (98.1% of discharged patients), of whom 258 (9.8%) had since been re‐admitted to hospital (234 unplanned, 24 planned admissions). Clinical frailty scores were recorded for 2701 patients prior to surgery (98.9%; mean score, 2.8; SD, 1.2) and for 2500 of 2614 patients alive at 30‐day follow‐up (95.6%; mean score, 2.8; SD, 1.3). Data were complete for 254 350 of 256 140 fields (99.3%; missing data, by variable: Supporting Information, table 2).
Investigation and management of anaemia prior to surgery
Haemoglobin levels prior to surgery were documented for 2461 of 2727 patients (90.2%; elective surgery, 1160 of 1411 patients [82.2%]; non‐elective surgery, 1298 of 1313 patients [98.9%]). For the patients whose levels were checked, the mean value was 131.0 g/L (SD, 18.9 g/L), and 689 had anaemia (28.0%) (Box 3).
The proportions of patients with pre‐operative anaemia who required pRBC transfusion during (8.1% v 1.2%) or after surgery (18% v 2.5%), experienced post‐surgical complications (40.4% v 21.1%), underwent re‐operation (5.4% v 2.4%), or had medical emergency team calls (8.9% v 3.4%) were larger than for those without anaemia, and their median length of hospital stay was longer (6 [IQR, 3–13] v 3 [IQR, 1–6] days) (Supporting Information, table 4).
Iron studies were performed for 243 patients with pre‐operative anaemia (35.3%), of whom 128 (53%) had ferritin levels below 100 μg/L and 65 (27%) below 30 μg/L (reference intervals: women [pre‐menopausal], 20–220 μg/L; women [post‐menopausal], 30–370 μg/L; men, 30–620 μg/L12). Forty‐one of 128 patients with iron deficiency anaemia (32%) received intravenous iron prior to surgery (Box 4).
Anaemia was thus fully investigated and managed in 1928 of 2727 patients prior to surgery (70.7%) (Box 4). In these patients, the proportions who received pRBC transfusions during (2.0% v 4.5%) or after surgery (4.1% v 11%), or experienced major complications (5.9% v 9.0%), were smaller and the median length of hospital stay shorter (3 [IQR, 1–7] v 4 [IQR, 1–9] days) than for the 798 patients for whom anaemia was not fully investigated and managed (Box 5). In multivariate analyses, pre‐operative anaemia assessment and management were associated with lower likelihood of intra‐operative (aOR, 0.33; 95% CI, 0.19–0.57) and post‐operative pRBC transfusion (aOR, 0.36; 95% CI, 0.25–0.53), and of post‐surgery complications (aOR, 0.79; 95% CI, 0.63–0.99) (Box 6).
Tranexamic acid administration during surgery
Tranexamic acid was administered during 128 of 2728 procedures (4.7%), including 34 of 153 gynaecological (22%) and 21 of 1223 hepatobiliary operations (1.7%). Tranexamic acid was most frequently administered to patients with pre‐operative haemoglobin levels below 80 g/L (seven of 26 patients, 27%) or undergoing immediate procedures (13 of 64 patients, 20%) (Supporting Information, table 5). Of the 78 patients who received pRBC transfusions during surgery (ie, who underwent procedures with potentially significant blood loss), 22 received tranexamic acid (28%). Tranexamic acid during surgery was not associated with significant differences in the likelihood of transfusion during or after surgery, post‐operative complications, or length of hospital stay (Box 5, Box 6).
Restrictive post‐operative transfusion strategy
Haemoglobin measurements prior to pRBC transfusion were available for each of the 167 of the 2728 patients who received transfusions after surgery (6.1%); the mean value was 73.9 g/L (SD, 11.6 g/L; range, 34–117 g/L). Sixty‐nine patients received one unit (41%), 43 two units (26%), 16 three units (10%), and 39 four or more units (23%). Restrictive transfusion guidelines were followed for 96 patients (58%), but were not associated with statistically significant differences in likelihood of post‐operative complications, or in length of stay (Box 5, Box 6).
Identification of anaemia after surgery
Haemoglobin was measured one to three days after surgery for 2161 of 2726 patients (79.3%); the mean nadir value was 113 g/L (SD, 20.0 g/L; range, 27–164 g/L), and anaemia was identified in 1479 patients (68.4% of those assessed) (Box 3). Haemoglobin was not assessed in 52 of 689 patients with anaemia prior to surgery (7.5%).
Excluding the 33 patients who died in hospital and the 29 not discharged by the censoring date, haemoglobin values were recorded for 2069 of 2635 patients prior to discharge (78.5%). The mean value was 118 g/L (SD, 17.5 g/L; range, 64–180 g/L), and anaemia was identified in 1227 patients (59.3%). The proportion of people with anaemia at discharge re‐admitted to hospital within 30 days of discharge was larger than for those discharged without anaemia (169 of 1207 patients followed up, 14.0% v 61 of 825, 7.4%). A larger proportion of people discharged with haemoglobin levels below 100 g/L were re‐admitted within 30 days (69 of 319 patients followed up, 22%) than of those with values of 100 g/L or more (161 of 1713, 9.4%).
Haemoglobin measurements 30 days after discharge were recorded for 472 of 2635 patients (17.9%); 255 had anaemia (54.0%). Of the 1227 patients with anaemia at discharge, follow‐up haemoglobin measurements were recorded in hospital records for 299 (24.4%), of whom 220 still had anaemia (73.6%) (Box 3).
Management of post‐operative anaemia
Oral iron was prescribed after surgery for six of 1227 patients with anaemia at discharge (0.5%) and seven of 842 patients without anaemia at discharge (0.9%). Intravenous iron was administered to 70 of 1227 patients with anaemia at discharge (5.7%) and to 39 of 322 patients with haemoglobin values below 100 g/L (12%).
Centre‐level variation
With the exception of post‐operative oral iron prescribing, adherence to audit standards varied significantly between hospitals (Supporting Information, figure 2).
Sensitivity analyses
In sensitivity analyses that excluded the 1112 patients who had undergone cholecystectomy, the proportion of patients whose haemoglobin levels were assessed prior to surgery (1476 of 1616 patients, 91.3%) was similar to that for the main analysis (90.2%), but the proportion with pre‐operative anaemia was larger (498 patients, 33.7% v 28.0%). The proportion of patients with pre‐operative anaemia in whom iron was investigated was larger (220 patients, 44.2% v 35.3%), but the proportion treated with intravenous iron was similar (40 patients, 35% v 32%). Tranexamic acid use during surgery and restrictive transfusion after surgery were similar in the sensitivity and main analyses. Haemoglobin was assessed after surgery in a larger proportion of patients in the sensitivity analysis (1449 of 1618 patients, 89.6% v 79.3%), and the proportion with anaemia at discharge was also larger (946 of 1368 patients, 69.2% v 59.3%) (Supporting Information, sensitivity analyses).
Discussion
Anaemia is common in patients undergoing major abdominal surgery in Australia and New Zealand. We found that most patients are investigated appropriately prior to surgery, and that adherence to guidelines was associated with reduced likelihood of both complications and blood transfusion. Conversely, most patients had anaemia at discharge, but few received appropriate management or follow‐up. Hospital re‐admission within 30 days was more frequent among people who had anaemia at discharge. Improving the identification and management of peri‐operative anaemia could improve patient outcomes after surgery.
National health authorities in Australia and New Zealand have adopted consensus guidelines for blood management in patients undergoing surgery.3,13 The per capita use of intravenous iron in Australia is among the highest in the world, causing costs to the Pharmaceutical Benefits Scheme of more than $110 million annually.14 We found that haemoglobin was assessed in 90% of patients before abdominal surgery, and that iron studies were undertaken for 35% of those with anaemia. Adherence to individual components of the audit standard varied with operative urgency, reflecting the impracticality of iron studies in patients requiring acute surgery. Although the pre‐operative use of intravenous iron has been widely discussed,6 it is relatively uncommon in Australia and New Zealand, including in elective surgery.
Post‐operative anaemia was common, as was poor follow‐up and management. Hospital re‐admission within 30 days of discharge was more frequent among people who had anaemia at discharge, suggesting that more comprehensive investigation and follow‐up of patients with anaemia is needed.15 Post‐operative anaemia — associated with reduced likelihood of re‐admission as a secondary endpoint in PREVENTT6 — could be a target for intravenous iron therapy, including after discharge from critical care.16
More than half the post‐operative blood transfusions in our study (58%) were consistent with restrictive transfusion guidelines. A recent systematic review of relevant trials concluded that such strategies are safe.17 The 2012 ANBA guidelines recommend that transfusions be reserved for patients with haemoglobin levels below 80 g/L,3 but we applied a cut‐off of 70 g/L, in line with recent clinical trials. Were a threshold of 80 g/L applied, 88% of post‐operative transfusions would have complied with a restrictive approach (data not shown). In any case, current practice is satisfactory, and the restrictive approach did not affect the outcomes we assessed. A restrictive transfusion strategy minimises the risks associated with blood products and reduces the number of units required, improving the use of a valuable resource without compromising patient outcomes.3,18 We did not find any significant differences in outcomes associated with adherence to the audit standard, perhaps reflecting a general shift from more liberal transfusion policies. Further trials may provide evidence that will assist patient selection in this regard.19
Routine peri‐operative tranexamic acid administration has not been adopted in Australia and New Zealand. In our study sample, only 5% of patients undergoing major abdominal surgery received it, predominantly patients who also needed intra‐operative blood transfusions and those with pre‐operative anaemia. Tranexamic acid was most frequently used in gynaecological and urological procedures. Large randomised controlled trials have reported the benefits of tranexamic acid in reducing mortality among people with trauma,20 post partum haemorrhage,21 or bleeding during cardiac or orthopaedic surgery;22 smaller trials have reported its value during abdominal surgery.23,24,25 The findings of the recent POISE‐3 trial indicate that prophylactic tranexamic acid reduces bleeding during non‐cardiac surgery, supporting its use for reducing peri‐operative blood loss.26
Limitations
The prospective collection of a large volume of data on consecutive patients over a short period of time by a network of medical students and trainees, maximising data ascertainment and quality, provided a valuable snapshot of surgical practice in New Zealand and Australia. However, the Hawthorne effect, inevitable in prospective clinical audits, may have affected our findings. Further, our observational study was susceptible to variable sampling bias in the different specialties and hospitals, which may have influenced the apparent variation in adherence to guidelines. Nevertheless, our investigation of peri‐operative anaemia and its management in patients undergoing major abdominal surgery probably reflects current practice in Australia and New Zealand. Follow‐up haemoglobin measurements in private health or primary care may have not been recorded in hospital medical records. However, anaemia was still prevalent among patients whose levels were assessed at 30 days. We did not assess blood loss during surgery because it is inconsistently recorded in clinical documents; its analysis was therefore not feasible for a multicentre student‐ and trainee‐led audit. The study period included COVID‐19‐related lockdowns in Australia and New Zealand, and their impact on the identification and management of peri‐operative anaemia is unknown.
Conclusion
Anaemia is common in patients undergoing major abdominal surgery and influences surgical outcomes. More than half the patients in our study had anaemia at discharge, and it was often poorly managed and followed up. People discharged with post‐operative anaemia were more frequently re‐admitted to hospital. The management of peri‐operative anaemia needs to be improved to enhance surgical outcomes.
Box 1 – Guideline‐based audit standards for peri‐operative patient blood management3,4
|
Audit standard |
Explanation |
Adherence with standard |
|||||||||||||
|
|
|||||||||||||||
|
Prior to surgery |
|
|
|||||||||||||
|
Pre‐operative anaemia should be identified and managed |
Pre‐operative anaemia should be identified, evaluated, and managed to minimise need for red blood cell transfusion. |
Composite endpoint: patients without anaemia; patients with anaemia without iron deficiency; patients with iron deficiency anaemia who received intravenous iron therapy. |
|||||||||||||
|
During surgery |
|
|
|||||||||||||
|
Tranexamic acid should be given if substantial blood loss is anticipated (volume that would induce anaemia requiring therapy) |
Intravenous tranexamic acid is recommended when risk of blood loss is high. |
Tranexamic acid use in patients who required intra‐operative blood transfusion. |
|||||||||||||
|
Following surgery |
|
|
|||||||||||||
|
Restrictive blood transfusion (ie, only for patients with severe anaemia) should be standard of care |
Check haemoglobin level before and after each unit of blood administered, with target of > 70 g/L (> 80 g/L in patients with cardiac disease). |
Patients with pre‐transfusion haemoglobin level below 70 g/L (80 g/L in patients with cardiac disease) prior to post‐operative blood transfusion. |
|||||||||||||
|
Post‐operative haemoglobin levels should be measured |
All patients who have undergone major surgery and who had pre‐operative anaemia or moderate to severe blood loss during surgery must be screened for anaemia after surgery. |
Patients whose haemoglobin level was measured in hospital after surgery. |
|||||||||||||
|
Oral iron should not be prescribed |
As early oral iron therapy is not clinically beneficial in patients with post‐operative anaemia, its routine use is not recommended. |
Patients discharged alive with post‐operative anaemia who are not prescribed oral iron. |
|||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 – Demographic characteristics and clinical factors for 2730 patients who underwent major abdominal surgery in 56 Australian and New Zealand hospitals, July 2021
|
Characteristic |
Value |
||||||||||||||
|
|
|||||||||||||||
|
All participants |
2730 |
||||||||||||||
|
Age (years), mean (SD) |
56.7 (17.3) |
||||||||||||||
|
Sex (women) |
1558 (57.1%) |
||||||||||||||
|
Ethnic background |
|
||||||||||||||
|
Aboriginal or Torres Strait Islander |
46 (1.7%) |
||||||||||||||
|
Māori |
115 (4.2%) |
||||||||||||||
|
Pacific Islander |
60 (2.2%) |
||||||||||||||
|
Asian |
236 (8.6%) |
||||||||||||||
|
European or other |
1768 (64.8%) |
||||||||||||||
|
Missing data |
505 (18.5%) |
||||||||||||||
|
Body mass index (kg/m2), mean (SD) |
30.1 (7.6) |
||||||||||||||
|
Missing data |
100 (3.7%) |
||||||||||||||
|
ASA physical status |
|
||||||||||||||
|
I–II |
1597 (58.5%) |
||||||||||||||
|
III–IV |
1115 (40.9%) |
||||||||||||||
|
V |
8 (0.3%) |
||||||||||||||
|
Missing data |
10 (0.4%) |
||||||||||||||
|
Other medical conditions |
|
||||||||||||||
|
Cardiac |
778 (28.5%) |
||||||||||||||
|
Respiratory |
465 (17.0%) |
||||||||||||||
|
Diabetes mellitus |
406 (14.9%) |
||||||||||||||
|
Neurological |
190 (7.0%) |
||||||||||||||
|
Liver disease |
100 (3.7%) |
||||||||||||||
|
Missing data |
1 (< 0.1%) |
||||||||||||||
|
Smoking |
|
||||||||||||||
|
Current |
463 (17.0%) |
||||||||||||||
|
Previous |
697 (25.5%) |
||||||||||||||
|
Never |
1549 (56.7%) |
||||||||||||||
|
Missing data |
21 (0.8%) |
||||||||||||||
|
Surgical specialty* |
|
||||||||||||||
|
Hepatopancreatobiliary |
1224 (44.8%) |
||||||||||||||
|
Colorectal |
760 (27.8%) |
||||||||||||||
|
Upper gastrointestinal |
436 (16.0%) |
||||||||||||||
|
Gynaecology |
153 (5.6%) |
||||||||||||||
|
Urology |
113 (4.1%) |
||||||||||||||
|
Transplantation |
32 (1.2%) |
||||||||||||||
|
Vascular |
(0.4%) |
||||||||||||||
|
Surgical urgency |
|
||||||||||||||
|
Immediate |
64 (2.3%) |
||||||||||||||
|
Urgent |
623 (22.8%) |
||||||||||||||
|
Expedited |
626 (22.9%) |
||||||||||||||
|
Elective |
1413 (51.8%) |
||||||||||||||
|
Missing data |
4 (0.1%) |
||||||||||||||
|
Duration of surgery (min), median (IQR) |
122 (86–197) |
||||||||||||||
|
|
|||||||||||||||
|
ASA = American Society of Anesthesiologists; IQR = interquartile range; SD = standard deviation. * A more detailed list, by surgery subtype, is provided in the Supporting Information, table 1. |
|||||||||||||||
Box 3 – Prevalence of anaemia in patients who underwent major abdominal surgery in 56 Australian and New Zealand hospitals, July 2021, from before surgery to 30 days after surgery*
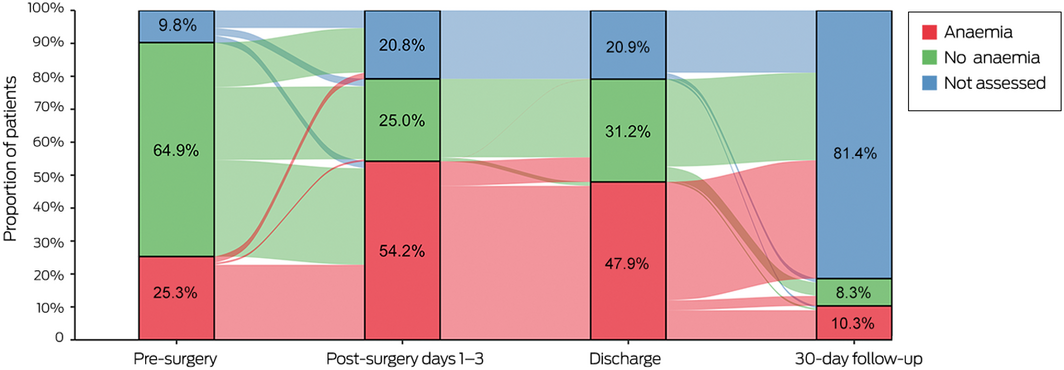
* The raw numbers underlying this graph are included in the Supporting Information, table 3. Ribbons between bars indicate change in assessment between time points.
Box 4 – Identification, investigation, and management of pre‐operative anaemia in patients who underwent major abdominal surgery in 56 Australian and New Zealand hospitals, July 2021*
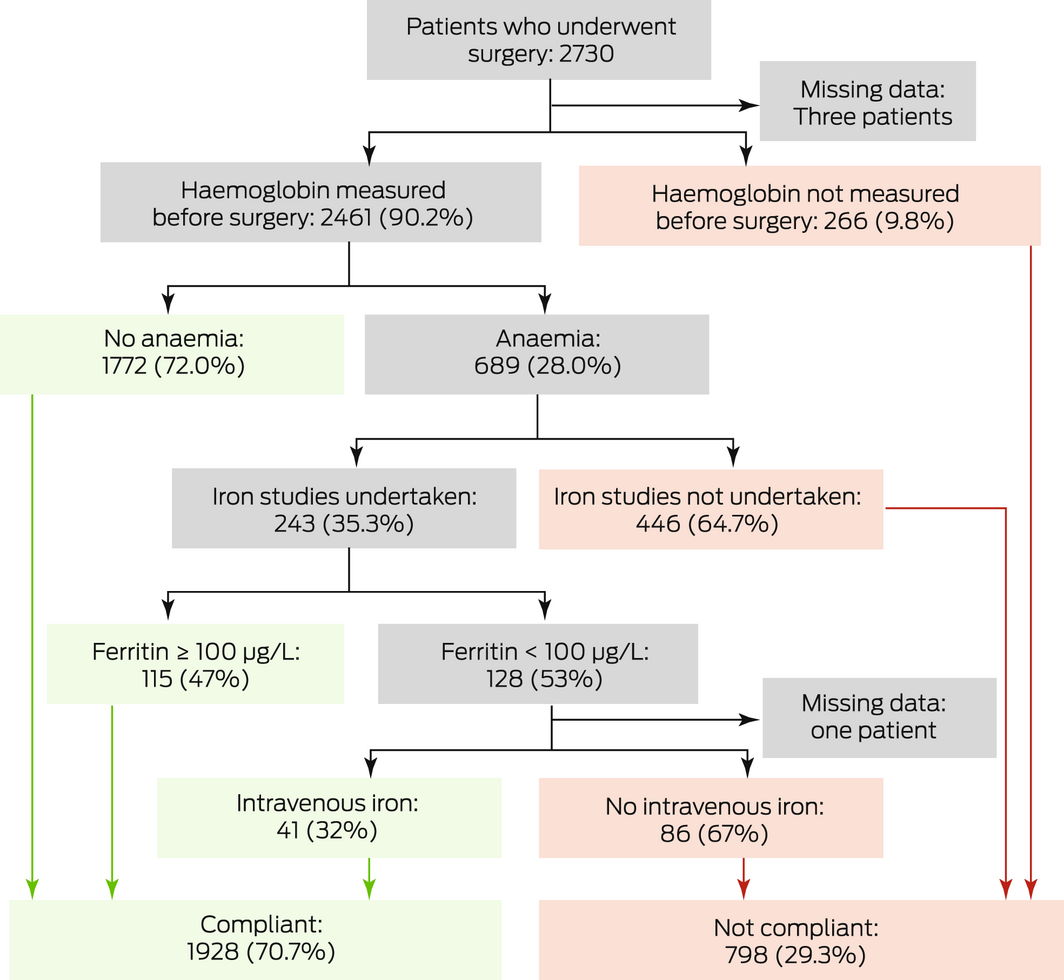
* Separate charts for patients undergoing elective surgery and non‐elective surgery are included in the Supporting Information, figure 1. Green boxes indicate compliance with the audit standard, red boxes non‐adherence.
Box 5 – Patient outcomes, by adherence to patient blood management audit standards
|
Audit guideline standard |
All patients |
Adherent |
Not adherent |
P |
|||||||||||
|
|
|||||||||||||||
|
Pre‐operative anaemia assessed/managed |
2726 |
1928 [70.7%] |
798 [29.3%] |
|
|||||||||||
|
pRBC units transfused during surgery |
|
|
|
0.008 |
|||||||||||
|
None |
2649 (97.0%) |
1888 (97.9%) |
757 (94.9%) |
|
|||||||||||
|
One |
20 (0.7%) |
10 (0.5%) |
10 (1.3%) |
|
|||||||||||
|
Two |
33 (1.2%) |
18 (0.9%) |
15 (1.9%) |
|
|||||||||||
|
Three or more |
25 (0.9%) |
11 (0.6%) |
11 (1.4%) |
|
|||||||||||
|
pRBC units transfused after surgery |
|
|
|
< 0.001 |
|||||||||||
|
None |
2561 (93.8%) |
1848 (95.9%) |
711 (89.1%) |
|
|||||||||||
|
One |
69 (2.5%) |
33 (1.7%) |
35 (4.4%) |
|
|||||||||||
|
Two |
43 (1.6%) |
19 (1.0%) |
24 (3.0%) |
|
|||||||||||
|
Three or more |
55 (2.0%) |
27 (1.4%) |
28 (3.5%) |
|
|||||||||||
|
Post‐surgery complications |
|
|
|
0.013 |
|||||||||||
|
None |
2058 (75.4%) |
1485 (77.0%) |
570 (71.4%) |
|
|||||||||||
|
Minor |
485 (17.8%) |
329 (17.1%) |
156 (19.5%) |
|
|||||||||||
|
Major* |
186 (6.8%) |
113 (5.9%) |
72 (9.0%) |
|
|||||||||||
|
Length of stay (days), median (IQR) |
3 (1–7) |
3 (1–7) |
4 (1–9) |
< 0.001 |
|||||||||||
|
Tranexamic acid used during surgery |
78 |
22 [28%] |
56 [72%] |
|
|||||||||||
|
pRBC units transfused during surgery |
|
|
|
0.92 |
|||||||||||
|
One |
20 (26%) |
4 (18%) |
16 (29%) |
|
|||||||||||
|
Two |
33 (42%) |
10 (46%) |
23 (41%) |
|
|||||||||||
|
Three or more |
25 (32%) |
8 (36%) |
17 (30%) |
|
|||||||||||
|
pRBC units transfused after surgery |
|
|
|
0.98 |
|||||||||||
|
None |
37 (47%) |
11 (50%) |
26 (46%) |
|
|||||||||||
|
One |
14 (18%) |
3 (14%) |
11 (20%) |
|
|||||||||||
|
Two |
7 (9%) |
1 (4%) |
6 (11%) |
|
|||||||||||
|
Three or more |
20 (26%) |
7 (32%) |
13 (23%) |
|
|||||||||||
|
Post‐surgery complications |
|
|
|
0.45 |
|||||||||||
|
None |
34 (43.6%) |
6 (27.3%) |
28 (50.0%) |
|
|||||||||||
|
Minor |
20 (25.6%) |
8 (36.4%) |
12 (21.4%) |
|
|||||||||||
|
Major* |
24 (30.8%) |
8 (36.4%) |
16 (28.6%) |
|
|||||||||||
|
Length of stay (days), median (IQR) |
12 (7–24) |
13 (7–28) |
11.5 (8–20) |
0.81 |
|||||||||||
|
Restrictive transfusion strategy |
167 |
96 [57%] |
71 [43%] |
|
|||||||||||
|
pRBC units transfused after surgery |
|
|
|
0.34 |
|||||||||||
|
One |
69 (41%) |
33 (34%) |
36 (51%) |
|
|||||||||||
|
Two |
43 (26%) |
27 (28%) |
16 (22%) |
|
|||||||||||
|
Three or more |
55 (33%) |
36 (38%) |
19 (27%) |
|
|||||||||||
|
Post‐surgery complications |
|
|
|
0.96 |
|||||||||||
|
None |
43 (26%) |
23 (24%) |
20 (28%) |
|
|||||||||||
|
Minor |
57 (34%) |
35 (36%) |
22 (31%) |
|
|||||||||||
|
Major* |
67 (40%) |
38 (40%) |
29 (41%) |
|
|||||||||||
|
Length of stay (days), median (IQR) |
13 (8–21.5) |
13 (8–21) |
12 (7–24) |
0.47 |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; pRBC = packed red blood cell. * Clavien–Dindo grade III or higher. |
|||||||||||||||
Box 6 – Patient outcomes, by adherence to patient blood management audit standards: multivariate analysis*
|
Characteristic |
Adjusted odds ratio (95% confidence interval) |
||||||||||||||
|
|
|||||||||||||||
|
Pre‐operative anaemia assessed |
|
||||||||||||||
|
Intra‐operative blood transfusion |
0.33 (0.19–0.57) |
||||||||||||||
|
Post‐operative blood transfusion |
0.36 (0.25–0.53) |
||||||||||||||
|
Post‐operative complication |
0.79 (0.63–0.99) |
||||||||||||||
|
Major post‐operative complication† |
0.71 (0.50–1.01) |
||||||||||||||
|
Length of stay (per day)‡ |
1.06 (0.96–1.16) |
||||||||||||||
|
Tranexamic acid used during surgery |
|
||||||||||||||
|
Post‐operative blood transfusion |
0.47 (0.11–1.84) |
||||||||||||||
|
Post‐operative complication |
3.52 (0.78–20.1) |
||||||||||||||
|
Major post‐operative complication† |
1.09 (0.21–5.48) |
||||||||||||||
|
Length of stay‡ |
1.18 (0.58–2.42) |
||||||||||||||
|
Restrictive transfusion strategy |
|
||||||||||||||
|
Post‐operative complication |
1.10 (0.46–2.64) |
||||||||||||||
|
Major post‐operative complication† |
0.53 (0.23–1.18) |
||||||||||||||
|
Length of stay‡ |
0.79 (0.52–1.19) |
||||||||||||||
|
|
|||||||||||||||
|
* Adjusted for age, sex, ethnic background, surgery type, surgical urgency. † Clavien–Dindo grade III or higher. ‡ Adjusted hazard ratio reported. |
|||||||||||||||
Received 13 March 2022, accepted 29 July 2022
- The POSTVenTT Study Collaborative*
Open access
Open access publishing facilitated by The University of Western Australia, as part of the Wiley – The University of Western Australia agreement via the Council of Australian University Librarians.
We thank the Royal Australasian College of Surgeons’ Clinical Trials Network Australia New Zealand (CTANZ) and Michael Lawrence‐Brown (University of Western Australia) for their support and funding of the POSTVenTT study.
No relevant disclosures.
- 1. Shander A, Knight K, Thurer R, et al. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med 2004; 116 (7 Suppl): 58S‐69S.
- 2. Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non‐cardiac surgery: a retrospective cohort study. Lancet 2011; 378: 1396‐1407.
- 3. Australian National Blood Authority. Patient blood management guidelines, module 2: perioperative. 2012. https://www.blood.gov.au/pbm‐module‐2 (viewed Dec 2021).
- 4. Muñoz M, Acheson AG, Bisbe E, et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018; 73: 1418‐1431.
- 5. Shand AW, Bell J, Henry A, et al. Rapid increase in intravenous iron therapy for women of reproductive age in Australia. Med J Aust 2020; 213: 85‐86. https://www.mja.com.au/journal/2020/213/2/rapid‐increase‐intravenous‐iron‐therapy‐women‐reproductive‐age‐australia
- 6. Richards T, Baikady RR, Clevenger B, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double‐blind, controlled trial. Lancet 2020; 396: 1353‐1361.
- 7. POSTVenTT Study Collaborators. Postoperative variations in anaemia treatment and transfusions (POSTVenTT): protocol for a prospective, multicentre, observational cohort study of anaemia after major abdominal surgery. Colorectal Dis 2022; 24: 228‐234.
- 8. von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344‐349.
- 9. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity (WHO/NMH/NHD/MNM/11.1). 2011. https://www.who.int/publications/i/item/WHO‐NMH‐NHD‐MNM‐11.1 (viewed Dec 2021).
- 10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205.
- 11. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489‐495.
- 12. Royal College of Pathologists of Australasia. Ferritin [RCPA manual, pathology tests]. 2015. https://www.rcpa.edu.au/Manuals/RCPA‐Manual/Pathology‐Tests/F/Ferritin#:~:text=Reference%20Interval%3A,30%2D370%20%C2%B5g%2FL (viewed Aug 2022).
- 13. Isbister JP. The paradigm shift in blood transfusion. Med J Aust 1988; 148: 306‐308.
- 14. Australian Department of Health. PBS expenditure and prescriptions report: 1 July 2020 to 30 June 2021. https://www.pbs.gov.au/statistics/expenditure‐prescriptions/2020‐2021/PBS_Expenditure_and_Prescriptions_Report_1‐July‐2020_to_30‐June‐2021.pdf (viewed Feb 2022).
- 15. Hands K, Taylor C, Kotzé A, et al. Preoperative patient blood management during the SARS‐CoV‐2 pandemic. Br J Haematol 2021; 193: 1087‐1092.
- 16. Shah A, Chester‐Jones M, Dutton SJ, et al; INTACT Investigators. Intravenous iron to treat anaemia following critical care: a multicentre feasibility randomised trial. Br J Anaesth 2022; 128: 272‐282.
- 17. Carson JL, Stanworth SJ, Alexander JH, et al. Clinical trials evaluating red blood cell transfusion thresholds: an updated systematic review and with additional focus on patients with cardiovascular disease. Am Heart J 2018; 200: 96‐101.
- 18. Ejaz A, Frank SM, Spolverato G, et al. Potential economic impact of using a restrictive transfusion trigger among patients undergoing major abdominal surgery. JAMA Surg 2015; 150: 625‐630.
- 19. Brierley RCM, Pike K, Miles A, et al. A multi‐centre randomised controlled trial of Transfusion Indication Threshold Reduction on transfusion rates, morbidity and healthcare resource use following cardiac surgery: study protocol. Transfus Apher Sci 2014; 50: 451‐461.
- 20. CRASH‐2 collaborators; Roberts I, Shakur H, Afolabi A, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH‐2 randomised controlled trial. Lancet 2011; 377: 1096‐1101.
- 21. Picetti R, Miller L, Shakur‐Still H, et al; WOMAN trial collaborators. The WOMAN trial: clinical and contextual factors surrounding the deaths of 483 women following post‐partum haemorrhage in developing countries. BMC Pregnancy Childbirth 2020; 20: 409.
- 22. Henry DA, Carless PA, Moxey AJ, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2011; CD001886.
- 23. Henry DA, Carless PA, Moxey AJ, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2011; CD001886.
- 24. Kumar S, Randhawa MS, Ganesamoni R, Singh SK. Tranexamic acid reduces blood loss during percutaneous nephrolithotomy: a prospective randomized controlled study. J Urol 2013; 189: 1757‐1761.
- 25. Molenaar IQ, Warnaar N, Groen H, et al. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta‐analysis. Am J Transplant 2007; 7: 185‐194.
- 26. Devereaux PJ, Marcucci M, Painter TW, et al; POISE‐3 Investigators. Tranexamic acid in patients undergoing noncardiac surgery. N Engl J Med 2022; 386: 1986‐1997.






Abstract
Objectives: To assess the prevalence and management of anaemia in patients undergoing major abdominal surgery, and the influence of guideline adherence on patient outcomes.
Design: Prospective observational cohort study.
Setting: 56 hospitals in Australia and New Zealand.
Participants: People aged 18 years or more who underwent major abdominal surgery during two 2‐week periods in July 2021.
Main outcome measures: Proportions of patients managed according to Australian National Blood Authority patient blood management guidelines. Secondary outcomes: anaemia prevalence, post‐operative complications, length of hospital stay, re‐admission within 30 days of discharge.
Results: Data were available for 2730 eligible patients (mean age, 56.7 years; SD, 17.3 years), including 1558 women (57.1%). Haemoglobin levels prior to surgery were documented for 2461 of 2727 patients (90.2%), 689 of whom had anaemia (28.0%). Pre‐operative anaemia assessment and management were associated with lower likelihood of intra‐operative (adjusted odds ratio [aOR], 0.33; 95% CI, 0.19–0.57) and post‐operative blood transfusion (aOR, 0.36; 95% CI, 0.25–0.53), and of post‐operative complications (aOR, 0.79; 95% CI, 0.63–0.99). Tranexamic acid was administered during 128 of 2728 procedures (4.7%); a restrictive transfusion strategy was followed for 96 of the 167 patients who received post‐operative blood transfusions (58%). Post‐operative anaemia was identified in 1227 of 2069 patients (59.3%) in whom haemoglobin was assessed prior to discharge. The proportion of people re‐admitted to hospital within 30 days was larger for patients with anaemia at discharge (169 of 1207 patients followed up, 14.0% v 61 of 825, 7.4%). Haemoglobin assessments were recorded by 30 days after discharge for only 288 patients with post‐operative anaemia (24.3%).
Conclusions: The management of peri‐operative anaemia differs between hospitals in Australia and New Zealand, with consequences for patient outcomes. Patients are often discharged after surgery with anaemia, which is therefore a potential therapeutic target.
Trial registration: Australian New Zealand Clinical Trials Registry, ACTRN12621001517864 (retrospective).