The global burden of injury is enormous. More lives are lost to injury than to malaria, tuberculosis, and the human immunodeficiency virus combined.1 Road traffic injuries alone comprise the leading cause of death among young adults, causing about 1.3 million deaths annually.2 Traumatic brain injury (TBI) contributes significantly to deaths and long term disability around the world,3,4,5,6 affecting about 69 million people each year.5 In Australia, the number of deaths caused by TBI, based on hospital admissions data, is high, but there are no national baseline data on outcomes after moderate to severe TBI.7,8,9
TBI is defined as injury to the brain caused by an external force.10 Causes of blunt TBI include road trauma, falls, and being struck by an object or person.11,12 The definition of moderate to severe TBI varies, but generally includes TBI leading to death, loss of consciousness for at least 30 minutes, post‐traumatic amnesia for at least 24 hours, a Glasgow Coma Scale (GCS) total score below 13 during the 24 hours following injury, or neuroimaging evidence of brain injury pathology.13
Parallel to the need for national baseline summary data on the incidence and outcomes of moderate to severe TBI in Australia is the need to determine the extent and sources of variation in patient outcomes. Specifically, we need to identify population subgroups with greater burden of moderate to severe TBI and the determinants of adverse outcomes. Further, the effects of variations in access to emergency care and being able to return home have not been examined. Finally, the outcomes and process indicators that matter most to people with TBI need to be established.
Studies of several patient‐level clinical interventions following moderate to severe TBI have reported only limited improvement of patient outcomes.14,15,16 Our focus must therefore shift to understanding how best to target, standardise, and optimise systems of care, and how these systems affect clinical outcomes. However, reducing the incidence of catastrophic outcomes after moderate to severe TBI will be very difficult without baseline data.
The Australian Traumatic Brain Injury National Data (ATBIND) project will identify the key determinants of priority outcomes for patients with moderate to severe TBI in Australia.17 ATBIND will collate national baseline data that will inform decisions about where to target patient‐ and system‐level care interventions and measures for preventing TBI, and for monitoring progress benchmarked against relevant measurable targets.17
Aims of the study
The overarching aim of the ATBIND project is to understand the key determinants (demographic characteristics, injury event, processes of care) of patient outcomes at hospital discharge following moderate to severe TBI; that is, to determine the crude (unadjusted) and independent (adjusted) prognostic factors for people with moderate to severe TBI admitted to Australian major trauma centres (or dying before reaching a major trauma centre) that predict outcomes at hospital discharge.
The specific aims of the ATBIND project are:
- to determine, for Australia and for certain subgroups (including Aboriginal and Torres Strait Islander people), the incidence of priority outcomes, including survival to discharge home;
- to identify the main patient‐ and system‐level determinants of survival and function at hospital discharge;
- to develop a priority list of the major patient‐ and system‐level predictors of death and disability at hospital discharge;
- to develop models predicting death or disability at hospital discharge;
- to measure, on the basis of existing and extended targeted data (eg, coronial data), the impact of differences in residential location, demographic characteristics, injury mechanism, system‐level processes of patient referral, transfer, pre‐hospital care, emergency department reception, and hospital care on hospital discharge destination; and
- to establish a data‐ and consensus‐based set of national clinical quality indicators, targeting key inadequacies (including for the health of Aboriginal and Torres Strait Islander people) and inconsistencies in patient‐ and system‐level interventions associated with adverse outcomes for people with moderate to severe TBI.
Methods and analysis
The ATBIND project will analyse Australia New Zealand Trauma Registry (ATR; https://atr.org.au) data, as well as coronial data from all Australian states and territories. The ATR documents the demographic characteristics, injury event description and severity, processes of care, and outcomes for people who required assessment and management for major injury, including TBI, at the 27 major trauma services in Australia; about 90% of all patients with moderate to severe TBI are managed in these centres.18 The National Coronial Information Service (NCIS) database includes information on deaths reported to coroners, and its data will supplement ATR data with records for people who died before reaching a major trauma service. Our project will thereby determine the regional incidence of moderate to severe TBI and examine system‐level variation in outcomes.
Data will be included in our analyses for any person admitted to one of the 27 participating major trauma services in Australia who satisfied the ATR inclusion criteria (Injury Severity Score [ISS] higher than 12, or died in hospital) and had moderate to severe TBI, defined in our study as an Abbreviated Injury Scale (AIS) (head) score higher than 2.12,19 People will also be included if they died before reaching a major trauma service (ie, they died in another hospital type or before arriving at a hospital) and details in the corresponding coronial report are consistent with moderate to severe TBI.
The primary research outcome for the ATBIND project will be survival to hospital discharge. Secondary outcomes will be hospital disposition destination (discharge to usual accommodation, discharge to somewhere other than usual accommodation) and death; hospital length of stay; ventilator‐free days; and health service cost. All outcomes will be measured at the point of hospital discharge. An additional non‐clinical (data quality) outcome will be the level of completeness of the key derived primary outcome predictor variables (risk factors), including variables relevant to Aboriginal and Torres Strait Islander communities.20,21
We will include as potential risk factors in our analyses age, sex, remoteness, comorbid conditions, injury event details, vital signs and consciousness state, pupil size and reactivity, time from injury to definitive (major trauma service) hospital, mode of pre‐hospital transport, interventions performed en route to the definitive hospital (eg, endotracheal intubation), time from arrival at definitive (trauma service) hospital to first computed tomography (CT) imaging of the brain and operative management, type of operative intervention, injury severity (ISS, AIS for head), support services used in hospitals (eg, Aboriginal Health Worker, social worker), hospital discharge diagnosis (International Classification of Diseases, tenth revision [ICD‐10] codes), and diagnosis in coronial report. We will undertake corresponding subgroup analyses for Aboriginal and Torres Strait Islander people, people living in rural and remote areas, and people aged 65 years or more. Health service cost data for each person will be obtained from the casemix or performance unit at each major trauma service (Box 1).
The ATBIND project will also assess variations in outcomes for subgroups, including Aboriginal and Torres Strait Islander people. We will adhere to Indigenous data sovereignty and governance principles by expanding the scope of ATR data collected to better capture essential information. Specifically, additional data and consultation will focus on the particular needs of Aboriginal and Torres Strait Islander communities, while ensuring that information is not generated or shaped in a manner that reinforces or exacerbates marginalisation and disenfranchisement.21,22,23,24 The ATBIND project will examine the impact of system‐level health treatment, including the role of Aboriginal Liaison Officers and Health Workers, integrated care, telehealth, and timely access to pre‐ and inter‐hospital transport services and processes. An Indigenous data governance committee will be established at the start of the ATBIND project to ensure early integration of the principles of Indigenous knowledges and Indigenous data sovereignty.21,22,23,24
Data analysis
Our investigation will encompass three broad phases, respectively determining the incidence, predictors, and quality indicators of patient outcomes.
During phase 1 (incidence), we will apply appropriate regression techniques (negative binomial or Poisson regression); the primary measure of association for the effect of year and other potential exposure variables will be the incidence rate ratio (Box 2).
During phase 2 (predictors), we will initially use exploratory logistic regression to determine the influence of univariable (crude or unadjusted) risk factors and prognostic subgroups on the likelihood of death before hospital discharge. Risk factors identified by univariable regression will then be assessed in multivariable regression analyses, thereby adjusting for confounding. We will identify a set of independent risk factors for which the association with the primary outcomes will be separately quantified, and then separately examined in each of the prognostic subgroups. Secondly, we will employ predictive logistic regression to develop parsimonious prediction models, splitting the ATR data into appropriate derivation and validation data subsets. Candidate variables will be selected and sequentially tested in the model according to the level of statistical significance of their association with the primary outcome. Further prediction models will be derived and validated in the same way for prognostic subgroups. The performance of the prediction models will be evaluated with conventional measures, including assessment of the area under the receiver operating characteristic (AUROC) curve.
In phase 3 (indicators), we will identify a suite of quality indicators using quasi‐Delphi methodology. Having established the identity and contributions of the key determinants of patient outcomes after moderate to severe TBI, we will use a consensus‐based, iterative focus group approach to determine national clinical quality indicators for monitoring processes of care and outcomes. To generate a list of SMART (specific, measurable, achievable, relevant, time‐bound) and consensus‐based quality indicators, key stakeholders will be recruited in the relevant clinical, research, and community groups, including Aboriginal and Torres Strait Islander organisations, health professionals, researchers, and senior community representatives relevant to the prognostic factors, care, and outcomes of people with moderate to severe TBI. This process will be essential to the effectiveness (including an understanding of cost‐effectiveness) and sustainable implementation of the ATBIND project, informing the targets and opportunities for improving outcomes after moderate to severe TBI.
The cost‐effectiveness of the quality indicators will be assessed in cost–consequence analyses. The additional cost of assessing clinical quality indicators to improve TBI outcomes will be estimated by calculating the marginal effect on health service cost of adherence to processes measured by each indicator. The additional cost associated with a clinical indicator (incremental cost) will be calculated using the recycled prediction method, varying the parameters included in the indicator while other parameters are held constant.25 The mean difference of the predictions, with and without the parameters included, will provide the incremental cost attributable to the clinical indicator. The cost–consequence analysis will compare estimates of the incremental cost and outcome of alternative clinical quality indicators to evaluate their relative cost‐effectiveness.
For phases 1 and 2, we will extract ATR and NCIS data. It is estimated that about 20 000 TBI cases during the five years since the establishment of the ATR in 2015 will meet our inclusion criteria.18 The first two phases of our investigation will be conducted over a period of about one year. For phase 3, the different participant groups (including Aboriginal and Torres Strait Islander people) will include TBI researchers and health care workers, as well as people with experience of TBI (patients, family, carers) to ensure that a meaningful and enduring set of quality indicators is derived. Each phase 3 group will include 15–25 participants. The phase 3 analyses will require about six months.
For descriptive statistics reports, symmetrical numerical data will be summarised as means with standard deviations, asymmetrical numerical and ordinal data as medians with interquartile ranges, and nominal data as counts and frequencies. For inferential analyses, we will assess the statistical significance of inter‐group differences in Student t, Wilcoxon rank sum, χ2, and Fisher exact tests as appropriate. P < 0.05 will be deemed statistically significant. Analyses will be performed in Stata 17.0.
Data storage
The data extracted or collected for analysis in the ATBIND project will be stored in Monash SeRP, the Monash University authorised secure platform.
Study timetable and site
The official start date for the ATBIND project was 1 June 2021, and the anticipated completion date is 31 May 2023. Phases 1 (incidence) and 2 (predictors) will be completed for all subgroups by 30 November 2022. Phase 3 (quality indicators) will be completed by 31 May 2023. The ATBIND project coordinating site is the National Trauma Research Institute, the Alfred Hospital, Melbourne, Victoria.
Ethics statement
The Alfred Ethics Committee approved ATR data extraction (project reference number 670/21; November 2021). Further ethics approval has been sought from the NCIS and multiple jurisdictional ethics approval bodies for research involving Aboriginal and Torres Strait Islander people.22
Dissemination of findings
The ATBIND project will determine the extent to which demographic characteristics, injury mechanism and severity, and treatment and system‐level interventions affect outcomes for Indigenous and other Australians with TBI. Further, the project will deliver meaningful and important outcomes for all Australians.17,26 Stakeholder representatives will be engaged in the dissemination of project findings, including the 27 trauma services who collaborate through the ATR and the Australian Trauma Quality Improvement Program (AusTQIP), as well as via the Australasian Trauma Society (ATS) and the Australasian Injury Prevention Network. Community peak body groups involved in this project, including Brain Injury Australia, Synapse (https://synapse.org.au) and the National Aboriginal Community Controlled Health Organisation (NACCHO), will also disseminate its findings. The Indigenous data governance committee will ensure the integration of Indigenous data sovereignty principles into the reporting and dissemination of project findings. Further, the work of the ATBIND project, guided by community consultation and the Indigenous data governance committee, will be integrated into the ongoing activities of the ATR (data collection, analysis, reporting) to help build a sound foundation for informing sustainable quality improvement initiatives and to provide an exemplar for other health registries for equitably gathering health data and analysing and reporting findings.
Author contributions
All authors contributed to the design of the protocol, including its concept and the critical review and final approval of the manuscript. Gerard O’Reilly led the initial drafting of the protocol. Mark Fitzgerald, Kate Curtis, Yesul Kim, Nick Rushworth, Biswadev Mitra, Jin Tee, Kate Hunter, Courtney Ryder, and Delia Hendrie continually reviewed the initial protocol. Ryder, Hunter and Shane D’Angelo further improved the protocol and manuscript, including the integration of Indigenous research excellence principles. Afsana Afroz further improved the design of the manuscript. O’Reilly, Fitzgerald, Curtis, Mitra, Kim, and Tee contributed to the protocol methodology, including the extraction and analysis of ATR data. Hendrie provided health economics expertise, O’Reilly and Mitra provided statistical consultancy, and Rushworth, Ryder, Hunter, and D’Angelo supported the development of community partnerships.
Funding statement
The ATBIND project, for which all protocol authors are either Chief Investigators or project research staff, is supported by a Medical Research Future Fund (MRFF) grant (MRF2007671; 1 June 2021).
The ATR receives support from the Australian government (Administration of the ATR, GO2992) and from non‐government sources (Accident Compensation Corporation, New Zealand).
Received 3 February 2022, accepted 7 June 2022
Box 1 – Study population and subgroups, outcomes, and potential determinants of outcomes to be investigated by the Australian Traumatic Brain Injury National Data (ATBIND) project
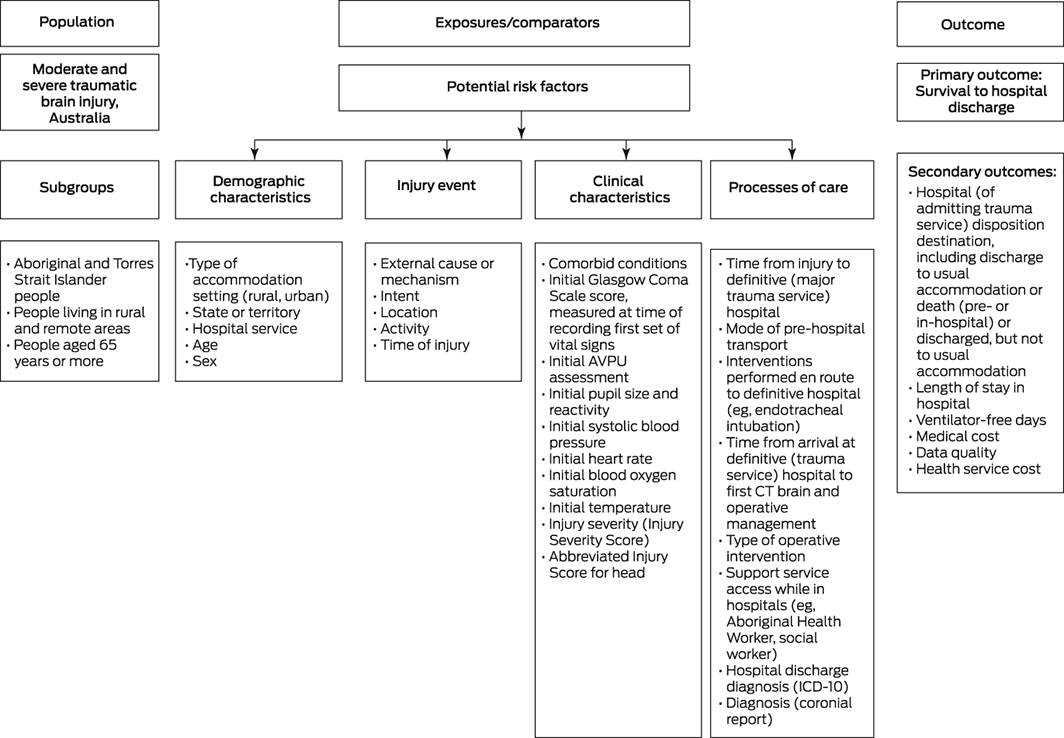
AVPU = alert, verbal, pain, unresponsive (consciousness scale); CT = computed tomography imaging; ICD = International Classification of Diseases, tenth revision.
Box 2 – Overview of the three phases of the Australian Traumatic Brain Injury National Data (ATBIND) project*
Phase 1. Incidence What is the incidence of moderate to severe traumatic brain injury (TBI), and what are the outcomes for patients?
- Project outcome 1 (incidence): Determination of the incidence of moderate and severe TBI and its outcomes, for Australia and for pre‐specified subgroups (including Aboriginal and Torres Strait Islander people).
- Study question 1: What is the incidence of moderate to severe TBI, what are the outcomes for patients, and how has the incidence changed over time? Population: all people with moderate to severe TBI; Exposure: most recent year; Comparator: preceding four years; Outcomes: primary, death; secondary, hospital discharge destination.
- Project outcome 2 (determinants): improved understanding of the main patient‐ and system‐level determinants of death and hospital discharge destination.
- Project outcome 3 (predictors): targeted priority list of the major patient‐ and system‐level predictors for death and hospital discharge destination.
- Project outcome 4 (prognostic models): development of models predicting pre‐hospital death and death and hospital discharge destination. Project outcome 5 (impact): measurement, based on existing and extended targeted data (including national coronial deaths data) of the impact of variations in demographic characteristics, injury mechanism, system‐level processes of patient referral, transfer, pre‐hospital care, emergency department reception, and hospital care on pre‐hospital death and hospital discharge destination.
- Study question 2: What are the prognostic factors and subgroups that influence likelihood of death and hospital discharge destination after moderate to severe TBI?
- Population: all people with moderate to severe TBI; Exposure: subgroup (eg, older people, Aboriginal or Torres Strait Islander people, rural location, mental health diagnosis, substance use); Comparator: comparable subgroups; Outcome: primary, death; secondary, hospital discharge destination.
- Project outcome 6 (quality indicators): data‐based set of national clinical quality indicators, targeting identified problem areas in health care (including for the health and wellbeing of Aboriginal and Torres Strait Islander people) and inconsistencies in patient‐ and system‐level interventions associated with differences in patient outcomes.
- Study question 3: What are the SMART (specific, measurable, attainable, relevant, time‐based) data‐ and consensus‐based national quality indicators required for monitoring the outcomes and processes of care for patients with moderate to severe TBI?
- Gerard M O'Reilly1,2,3
- Kate Curtis4,5,6,7
- Yesul Kim1,8
- Biswadev Mitra1,2,3
- Kate Hunter7
- Courtney Ryder7,9
- Delia V Hendrie10
- Nick Rushworth11
- Afsana Afroz1
- Shane D'Angelo9
- Jin Tee1,2,8
- Mark C Fitzgerald1,2,8
- 1 National Trauma Research Institute, Alfred Hospital, Melbourne, VIC
- 2 Alfred Health, Melbourne, VIC
- 3 Monash University, Melbourne, VIC
- 4 Sydney Nursing School, University of Sydney, Sydney, NSW
- 5 Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, NSW
- 6 Wollongong Hospital, Illawarra Shoalhaven Local Health District, Wollongong, NSW
- 7 The George Institute for Global Health, Sydney, NSW
- 8 Central Clinical School, Monash University, Melbourne, VIC
- 9 Flinders University, Adelaide, SA
- 10 Curtin University, Perth, WA
- 11 Brain Injury, Australia, Sydney, NSW
Open access
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
The following organisations have contributed significantly to the design of the protocol or its implementation: the National Trauma Research Institute (NTRI), the Alfred Hospital and Monash University (Melbourne), the Australia New Zealand Trauma Registry (ATR), the George Institute for Global Health, the University of Sydney, Brain Injury Australia, Flinders University (Adelaide), Curtin University (Perth), Synapse, and the National Aboriginal Community Controlled Health Organisation (NACCHO).
No relevant disclosures.Received 3 February 2022, accepted 7 June 2022
- 1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204‐1222.
- 2. World Health Organization. Road traffic injuries. 20 June 2022. https://www.who.int/news‐room/fact‐sheets/detail/road‐traffic‐injuries#:~:text=Road%20traffic%20injuries%20are%20the,result%20of%20road%20traffic%20crashes (viewed July 2022).
- 3. Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol 2019; 18: 24‐25.
- 4. Centers for Disease Control and Prevention. TBI data. Updated 11 May 2021. https://www.cdc.gov/traumaticbraininjury/data/tbi‐edhd.html (viewed Dec 2021).
- 5. Dewan MC, Rattani A, Gupta S, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg 2018; 130: 1080‐1097.
- 6. Hyder AA, Wunderlich CA, Puvanachandra P, et al. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007; 22: 341‐353.
- 7. O’Connor P. Hospitalisation due to traumatic brain injury. Australia 1997–98 (Cat. no. INJCAT 43; Injury Research and Statistics, number 11). Canberra: Australian Institute of Health and Welfare, 2002. https://www.aihw.gov.au/reports/injury/hospitalisation‐traumatic‐brain‐injury‐1997‐98 (viewed Dec 2021).
- 8. Fortune N, Wen X. The definition, incidence and prevalence of acquired brain injury in Australia (Cat. no. DIS 15). Canberra: Australian Institute of Health and Welfare, 1999. https://www.aihw.gov.au/reports/disability/definition‐incidence‐prevalence‐of‐brain‐injury‐au (viewed Dec 2021).
- 9. Pozzato I, Tate RL, Rosenkoetter U, Cameron ID. Epidemiology of hospitalised traumatic brain injury in the state of New South Wales, Australia: a population‐based study. Aust N Z J Public Health 2019; 43: 382‐388.
- 10. Menon DK, Schwab K, Wright DW, Maas AI; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010; 91: 1637‐1640.
- 11. Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 2013; 9: 231‐236.
- 12. Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci 2018; 62: 535‐541.
- 13. Malec JF, Brown AW, Leibson CL, et al. The Mayo classification system for traumatic brain injury severity. J Neurotrauma 2007; 24: 1417‐1424.
- 14. Cooper DJ, Nichol AD, Bailey M, et al; POLAR Trial Investigators and the ANZICS Clinical Trials Group. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized clinical trial. JAMA 2018; 320: 2211‐2220.
- 15. Cooper DJ, Rosenfeld JV, Murray L, et al; DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011; 364: 1493‐1502.
- 16. Nichol A, French C, Little L, et al; EPO‐TBI Investigators; ANZICS Clinical Trials Group. Erythropoietin in traumatic brain injury (EPO‐TBI): a double‐blind randomised controlled trial. Lancet 2015; 386: 2499‐2506.
- 17. O’Reilly GM, Curtis K, Kim Y, et al. Establishing determinants and quality indicators for getting home alive following moderate to severe traumatic brain injury: the Australian Traumatic Brain Injury National Data Project. Emerg Med Australas 2021; 33: 1121‐1123.
- 18. Fitzgerald MC, Curtis K, Cameron PA, et al; AusTQIP Consortium. The Australian trauma registry. ANZ J Surg 2019; 89: 286‐290.
- 19. Savitsky B, Givon A, Rozenfeld M, et al. Traumatic brain injury: it is all about definition. Brain Inj 2016; 30: 1194‐1200.
- 20. The Australian Trauma Quality Improvement Program. Bi‐National Trauma Minimum Dataset (BNTMDS) for Australia and New Zealand. Core data items, data dictionary; version 1.51. Sept 2018. https://static1.squarespace.com/static/5b761ed3f93fd491065f7839/t/5bfb68231ae6cf43c2b9aad8/1543202855072/BNTMDS_v1.51.pdf (viewed Dec 2021).
- 21. Ryder C, Mackean T, Coombs J, et al. Indigenous research methodology: weaving a research interface. International Journal of Social Research Methodology 2020; 23: 255‐267.
- 22. Ryder C, Wilson R, D’Angelo S, et al. Indigenous data sovereignty and governance: the Australian Traumatic Brain Injury National Data project. Nat Med 2022; 28: 888‐889.
- 23. Ryder C, Mackean T, Hunter K, et al. Factors contributing to longer length of stay in Aboriginal and Torres Strait Islander children hospitalised for burn injury. Inj Epidemiol 2020; 7: 52.
- 24. Knight HE, Deeny SR, Dreyer K, et al. Challenging racism in the use of health data. Lancet Digit Health 2021; 3: e144‐e146.
- 25. Polinder S, Toet H, Panneman M, van Beeck E (eds). Methodological approaches for cost–effectiveness and cost–utility analysis of injury prevention measures. Geneva: World Health Organization, 2011. https://www.euro.who.int/__data/assets/pdf_file/0007/144196/e95096.pdf (viewed July 2022).
- 26. Australian Department of Health. Traumatic brain injury mission. Updated 29 Mar 2022. https://www.health.gov.au/initiatives‐and‐programs/traumatic‐brain‐injury‐mission (viewed Mar 2022).






Abstract
Background: Traumatic brain injury (TBI) is the largest contributor to death and disability in people who have experienced physical trauma. There are no national data on outcomes for people with moderate to severe TBI in Australia.
Objectives: To determine the incidence and key determinants of outcomes for patients with moderate to severe TBI, both for Australia and for selected population subgroups, including Aboriginal and Torres Strait Islander Australians.
Methods and analysis: The Australian Traumatic Brain Injury National Data (ATBIND) project will analyse Australia New Zealand Trauma Registry (ATR) data and National Coronial Information Service (NCIS) deaths data. The ATR documents the demographic characteristics, injury event description and severity, processes of care, and outcomes for people with major injury, including TBI, assessed and managed at the 27 major trauma services in Australia. We will include data for people with moderate to severe TBI (Abbreviated Injury Scale [AIS] (head) score higher than 2) who had Injury Severity Scores [ISS] higher than 12 or who died in hospital. People will also be included if they died before reaching a major trauma service and the coronial report details were consistent with moderate to severe TBI. The primary research outcome will be survival to discharge. Secondary outcomes will be hospital discharge destination, hospital length of stay, ventilator‐free days, and health service cost.
Ethics approval: The Alfred Ethics Committee approved ATR data extraction (project reference number 670/21). Further ethics approval has been sought from the NCIS and multiple Aboriginal health research ethics committees. The ATBIND project will conform with Indigenous data sovereignty principles.
Dissemination of results: Our findings will be disseminated by project partners with the aim of informing improvements in equitable system‐level care for all people in Australia with moderate to severe TBI.
Study registration: Not applicable.