SGLT2 inhibitors show promise in improving metabolic and cardiovascular health in type 1 diabetes, yet ideal candidates for therapy require careful selection
The development of sodium–glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin, dapagliflozin, canagliflozin and ertugliflozin, has been a true game changer in the treatment of type 2 diabetes. Landmark trials, such as EMPA‐REG OUTCOME, demonstrated the undisputed and impressive cardiorenal benefits of this medication class in individuals with type 2 diabetes.1,2 Interestingly, these life‐saving benefits were discovered almost accidentally, thanks to the United States Food and Drug Administration (FDA), which in 2008 mandated that all new type 2 diabetes medications should be subject to cardiovascular outcome trials before entering clinical practice.3
SGLT2 inhibitors now belong in the formulary kit of physicians beyond just endocrinologists. The indications for SGLT2 inhibitors recently expanded to include heart failure with reduced ejection fraction and kidney disease, even in individuals without diabetes — an unsurprising eventuality given that their mechanism of action is largely independent of insulin and glycaemic status. Their glucose‐lowering action occurs via inhibition of renal tubular glucose reabsorption leading to glucosuria. The mechanisms for cardiorenal protection likely include improved glycaemia, insulin resistance, reduced plasma urate levels, and lower epicardial fat volume. These lead to reduced cardiac inflammation and greater contractility and natriuresis‐related haemodynamic effects, which affect blood pressure, intravascular volume, sympathetic activation and tubuloglomerular feedback.4 Also owing to these actions, SGLT2 inhibitors are associated with increased risk of genitourinary infection (including necrotising fasciitis of the perineum) and acute kidney injury. They should therefore be avoided in individuals at risk of severe or recurrent genital infection, hypovolaemia or ketoacidosis.5 However, with such an array of direct and downstream mechanistic actions, it is no wonder that there are expectations that this drug class may find indications beyond type 2 diabetes.
SGLT2 inhibitors in type 1 diabetes: a logical therapeutic choice
For the 420 million people living with diabetes globally (including 1.2 million Australians), heart disease is the leading causes of death.6 In type 1 diabetes the risk is up to ten times that of the general population in the context of poor glycaemic control but remains double even with on‐target glycaemia.7 Increased cardiovascular risk is not fully explained by traditional vascular risk factors such as dysglycaemia, dyslipidaemia, hypertension and smoking. The unexplained risk gap has been proposed to be due to insulin resistance, a key pathophysiological factor in type 2 diabetes.8,9
Individuals with type 1 diabetes have been shown to have greater insulin‐resistance compared with their peers without diabetes.10 This likely relates to the non‐physiological subcutaneous route of insulin delivery, compounded by weight gain and abdominal adiposity, which is partially insulin‐induced and in excess of global obesity trends.11 Up to 30% of Australians with type 1 diabetes display metabolic syndrome features, a possible marker of insulin resistance.12 This issue is under‐recognised but potentially targetable with repurposed medications intended for type 2 diabetes used adjunctively with insulin, such as SGLT2 inhibitors.
SGLT2 inhibitors in type 1 diabetes: therapeutic and regulatory inertia
The envisaged metabolic benefits of SGLT2 inhibition in type 1 diabetes were demonstrated across three phase 3 clinical trial programs: EASE (empagliflozin), DEPICT (dapagliflozin), and inTandem (sotagliflozin, a dual SGLT1/2 inhibitor).13 Weight loss is usually in the order of 2–3 kg and glycated haemoglobin levels typically improve by up to 0.5%; importantly, without increased hypoglycaemia risk.13 Recognising these benefits, the European Medicines Agency (EMA) approved dapagliflozin 5 mg in March 2019 as an adjunct in the treatment of individuals with type 1 diabetes with overweight.14 Subsequent real‐world studies from Europe found improved glycaemia without the expected increase in insulin dose that is required to achieve normoglycaemia, and without the weight gain that is usually observed with treatment intensification.15 While the EMA’s decision was celebrated by diabetologists across the globe, support was not replicated by the FDA, which declined applications for dapagliflozin and empagliflozin largely due to risk concerns.16
Diabetic ketoacidosis (DKA), a serious diabetic emergency necessitating hospital management, is the main factor hindering regulatory approval of SGLT2 inhibitors in type 1 diabetes. The negative caloric balance created by drug‐induced glucosuria promotes ketone generation; yet ketosis can occur without hyperglycaemia, making detection more difficult. Euglycaemic ketoacidosis is reported in patients with type 2 diabetes treated with SGLT2 inhibitors, with severe illness, prolonged fasting, and carbohydrate restriction; the peri‐procedural period is identified as a setting necessitating temporary drug cessation.5 Concerns regarding ketosis risk are justifiably greater in populations with insulin deficiency.
In clinical trials, DKA risk was dose‐dependent and not evident in participants treated with very low doses of SGLT2 inhibitors (dapagliflozin 5 mg daily for 24 weeks, empagliflozin 2.5 mg daily).13 It is unclear whether other benefits, such as cardiorenal protection would carry forward at doses lower than those in the major cardiovascular outcome trials. DKA is considered likely artificially low in clinical trials, which are typically resourced to provide intensive education. In response, consensus guidelines specific to type 1 diabetes were devised to mitigate DKA risk.17,18 EMA approval of dapagliflozin was conditional on mandatory provision of a strict risk mitigation strategy by prescribers. Reassuringly, early real‐world evidence from European registry data showed no increase in DKA risk.15 In light of the initial enthusiasm and safety of low dose SGLT2 inhibitors in type 1 diabetes, we and others were concerned to learn that EMA approval was abruptly reversed in October 2021.19,20 The diabetes community was reassured that this decision did not relate to safety in type 1 diabetes, but rather to manage confusion on product information interpretation of DKA risk in type 1 diabetes versus other populations, reflecting instead a possible commercial decision.
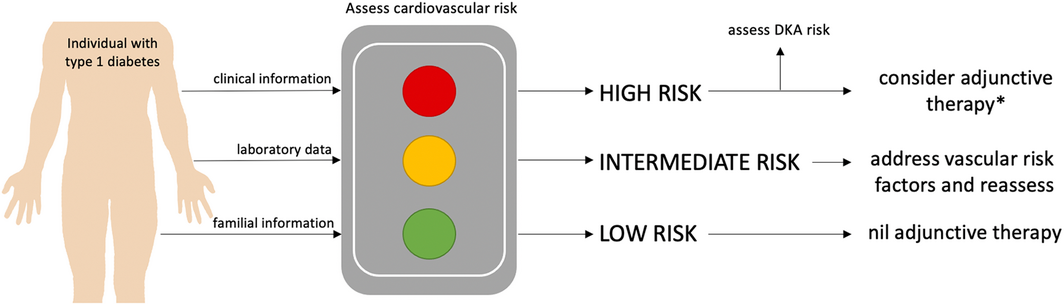
This brings us to the core issues in type 1 diabetes research and innovation that challenge our capacity to study medications that address cardiovascular risk. The distinct pathophysiology, earlier age of disease onset, metabolic environment (hypoglycaemia and ketosis risk) and unique drivers of insulin resistance compel type 1 diabetes‐specific study. With lower disease prevalence relative to type 2 diabetes, research resources are often funnelled accordingly. The conduct of cardiovascular outcome trials necessitates large sample sizes across long periods to capture hard clinical endpoints. In fact, cardiovascular outcome trials in type 2 diabetes have amassed over 200 000 volunteers to participate in various drug development programs. This degree of resource investment is unmatched in type 1 diabetes research. The requirement to enrol participants with high cardiovascular risk or established cardiovascular disease further hinders recruitment potential. Additionally, the same FDA mandate for cardiovascular outcome trials does not exist in type 1 as it did in type 2 diabetes, meaning there is less incentive or likelihood that cardiovascular outcome trials may ever eventuate.13 The only randomised controlled trial to measure mortality impacts of an intervention in type 1 diabetes was a landmark study, published almost 30 years ago, which demonstrated the benefits of intensive insulin therapy.21 Strict glucose targets then became the standard of care, but not without the sequelae of insulin therapy discussed above, including weight gain and hypoglycaemia.22 In light of the compelling and robust evidence for cardiorenal protection in type 2 diabetes, the absence of very large randomised controlled trials is an insufficient reason to exclude the use of SGLT2 inhibitors for metabolic benefit in type 1 diabetes. Novel solutions could include adaptive trial designs to allow for restricted clinical trial sample sizes, but there may be scope for more selective approval by regulatory bodies and the introduction of risk–benefit estimation tools. Indeed, a targeted approach to prescription of SGLT2 inhibitors in those most likely to benefit (such as individuals with glycated haemoglobin levels > 8% and higher body mass index) seems sensible.23 Combined with type 1 diabetes‐specific cardiovascular risk estimator tools that compute risk based on multiple inputs (age, diabetes duration, blood pressure, glycaemia, albuminuria), there is opportunity to select ideal risk–benefit candidates (Box).24 This may provide a compromise solution without current access to hard cardiovascular outcome data in type 1 diabetes. Equally, groups at greatest risk of DKA could be avoided, including women aged 25–44 years, captured by registry data reporting off‐label SGLT2 inhibitor use in the United States.25
In Australia, we have watched on in recent years, hopeful that SGLT2 inhibitors would achieve approval for type 1 diabetes across the globe, and that Australian Therapeutic Goods Administration approval would follow. The decision to withdraw dapagliflozin from the European Union likely caused ripples of lost confidence in approval boards internationally. In our experience, off‐label prescription of SGLT2 inhibitors is prevalent in Australia and is likely to continue. Therapeutic Goods Administration approval itself provides avenues to guide patient selection to reduce adverse effects and to support prescribers to confidently prescribe using appropriate DKA risk mitigation protocols.17 The ideal insulin adjunct in type 1 diabetes would achieve the goals of reduced hyperglycaemia, weight reduction, delay in progression of diabetes‐related complications, cardiorenal protection and mortality reduction, all without increased ketosis or hypoglycaemia. With careful patient selection, the use of low dose SGLT2 inhibitors may come close to fulfilling these goals. We envisage that a personalised approach based on assessment of cardiovascular risk is the future of type 1 diabetes management (Box). Without backing from both industry and regulators and a creative approach to research development and resource consumption, this ground‐breaking drug class will remain a lost opportunity to close the mortality gap in type 1 diabetes. We feel that solving this predicament is a very worthy investment.
Provenance: Not commissioned; externally peer reviewed.
- 1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117‐2128.
- 2. Giugliano D, Longo M, Scappaticcio L, et al. SGLT‐2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta‐analysis of 11 CVOTs. Cardiovasc Diabetol 2021; 20: 1‐11.
- 3. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry: Diabetes mellitus – evaluating cardiovascular risk and new antidiabetic therapies to treat type 2 diabetes. 2008. https://www.fda.gov/media/71297/download (viewed Jan 2022).
- 4. Ali A, Bain S, Hicks D, et al. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c – translating evidence into practice. Diabetes Ther 2019; 10: 1595‐1622.
- 5. Milder TY, Stocker SL, Day RO, Greenfield JR. Potential safety issues with use of sodium‐glucose cotransporter 2 inhibitors, particularly in people with type 2 diabetes and chronic kidney disease. Drug Saf 2020; 43: 1211‐1221.
- 6. Australian Institute of Health and Welfare. Deaths among people with diabetes in Australia, 2009–2104 (Cat. no. CVD 79). Canberra: AIHW, 2017. https://www.aihw.gov.au/getmedia/c164ec7c‐fc66‐4991‐8e53‐62eba274ead4/aihw‐cvd‐79.pdf.aspx?inline=true (viewed Jan 2022).
- 7. Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014; 371: 1972‐1982.
- 8. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014; 10: 293‐302.
- 9. Schauer IE, Snell‐Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin‐mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes. Diabetes 2011; 60: 306‐314.
- 10. Greenfield JR, Samaras K, Chisholm DJ. Insulin resistance, intra‐abdominal fat, cardiovascular risk factors, and androgens in healthy young women with type 1 diabetes mellitus. J Clin Endocrinol Metab 2002; 87: 1036‐1040.
- 11. Van der Schueren B, Ellis D, Faradji RN, et al. Obesity in people living with type 1 diabetes. Lancet Diabetes Endocrinol 2021; 9: 776‐785.
- 12. Lee AS, Twigg SM, Flack JR. Metabolic syndrome in type 1 diabetes and its association with diabetes complications. Diabetic Med 2021; 38: e14376.
- 13. Snaith JR, Holmes‐Walker DJ, Greenfield JR. Reducing type 1 diabetes mortality: role for adjunctive therapies? Trends Endocrinol Metabol 2020; 31: 150‐164.
- 14. European Medicines Agency. First oral add‐on treatment to insulin for of certain patients with type 1 diabetes [media release]. 1 Feb 2019. https://www.ema.europa.eu/en/news/first‐oral‐add‐treatment‐insulin‐treatment‐certain‐patients‐type‐1‐diabetes (viewed Jan 2022).
- 15. Seufert J, Lanzinger S, Danne T, et al. Real‐world data of 12‐month adjunct sodium‐glucose co‐transporter‐2 inhibitor treatment in type 1 diabetes from the German/Austrian DPV registry: improved HbA1c without diabetic ketoacidosis. Diabetes Obes Metab 2022; 24: 742‐746.
- 16. Ault A. FDA panel rejects empagliflozin for use in type 1 diabetes. Medscape Medical News 2019; 14 Nov. http://www.medscape.com/viewarticle/921303 (viewed June 2022).
- 17. Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther 2018; 20: 571‐575.
- 18. Danne T, Garg S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium–glucose cotransporter (SGLT) inhibitors. Diabetes Care 2019; 42: 1147‐1154.
- 19. European Medicines Agency. Forxiga (dapagliflozin) 5mg should no longer be used for the treatment of type 1 diabetes mellitus. https://www.ema.europa.eu/en/medicines/dhpc/forxiga‐dapagliflozin‐5mg‐should‐no‐longer‐be‐used‐treatment‐type‐1‐diabetes‐mellitus (viewed Jan 2022).
- 20. Czupryniak L, Danne T, Szymańska‐Garbacz E, et al. SGLT‐2 inhibitor for type 1 diabetes – not any more? Diabetes Obes Metab 2022; 24: 764‐765.
- 21. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977‐986.
- 22. Purnell JQ, Braffett BH, Zinman B, et al. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2017; 40: 1756‐1762.
- 23. Palanca A, van Nes F, Pardo F, et al. Real‐world evidence of efficacy and safety of SGLT2 inhibitors as adjunctive therapy in adults with type 1 diabetes: a European two‐center experience. Diabetes Care 2022; 45: 650‐658.
- 24. Steno Diabetes Center Copenhagen. The Steno T1 risk engine. https://www.sdcc.dk/english/research/projects/Pages/The‐Steno‐T1‐Risk‐Engine.aspx (viewed Apr 2022).
- 25. Hampp C, Swain RS, Horgan C, et al. Use of sodium–glucose cotransporter 2 inhibitors in patients with type 1 diabetes and rates of diabetic ketoacidosis. Diabetes Care 2020; 43: 90‐97.






Open access
Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
No relevant disclosures.