Implementation science based health care research is urgently needed for genomic and precision medicine in Australia
Precision medicine is a tailored approach to health, incorporating an individual's genetic make‐up, environment and lifestyle, and is a new frontier offering much promise for disease prevention and cure.1 Its recent rise has been largely driven by rapid advances in genomic medicine, with sequencing of an individual's genetic code identifying opportunities for precision health care, therapies and diagnostics. Genomics has revolutionised many areas, including public health (eg, population genetic carrier screening and pathogen genomic sequencing during the coronavirus disease 2019 [COVID‐19] pandemic), pharmacogenomics (drug metabolism and response genes), cancer management (tumour sequencing for diagnosis and therapy targets), pregnancy management (testing, screening and pre‐implantation genetic diagnosis) and rare diseases (Box 1). Medicare item numbers are now approved for genomic diagnostics in cancer, pre‐implantation genetic diagnosis, and certain paediatric, renal and cardiac conditions. New genetic therapies have arisen, including Therapeutic Goods Administration (TGA)‐funded ocular (voretigene neparvovec; Luxturna, Novartis), neuromuscular (onasemnogene abeparvovec; Zolgensma, Novartis), oncological (tisagenlecleucel; Kymriah, Novartis) and other genetic therapies.
It is an exciting time for genomic and precision medicine in Australia. An ever‐increasing proportion of families are receiving accurate genetic diagnoses, access to screening and counselling, and clinical management from publicly funded genomic technologies (Box 1), with other areas under investigation and a focus towards future government funding.10,11 However, despite the clinical benefits of genomics, the uptake in the clinic and bedside for patient care to access publicly funded new diagnostics and therapies is far from equitable or routine in Australia.12 Many challenges and barriers are known, with others yet to be documented (Box 1). At the clinician level, many non‐genetics professionals are not well prepared to use the newly funded genomic diagnostic tests. Medical and training curricula covering genetics and genomics require updating, including guidance from professional bodies and colleges, both in primary care and specialty groups such as ophthalmology.13,14 Even though many clinicians report they would rather refer to local genetics services or professionals to perform genomic testing, interpretation and clinical management of cases, the current genetics workforce in Australia is inadequate, with only an estimated 150 genetic physicians and 220 genetic counsellors in the country.15 Many clinical service waitlists have expanded to years rather than months.16 This reflects a significant worldwide issue, with up to 44% shortfall in the genetics workforce.17 At an organisation level, health care systems are struggling to adopt new genomic innovations, even when there is proven validity and utility.18
The translation gap between medical evidence‐based practices and actual clinical adoption is well recognised8 (Box 2). Genomic medicine and its contribution to precision medicine presents a unique set of challenges to a health system trying to keep up with the fast pace of advances over the past decade. An average of 17 years is required to integrate evidence‐based practices into routine health care, and genomics has exploded from widespread sequencing availability to TGA‐approved therapies requiring a precise genetic diagnosis in less than a decade.20 However, gaps in evidence, adoption, equity, and models of care remain, which have an impact on quality of care, cost effectiveness and resource utilisation12 (Box 1).
Genomic implementation: challenges ahead for Australia's precision medicine program
In a 2021 report1 on the new frontier of health in Australia, nationwide access to genomic testing and genomic counselling for all patients was recommended, but significant implementation barriers such as lack of genetics workforce were not addressed. Regulatory authority recommendations were made for improvement of availability of precision therapies, but key challenges in the adoption of genomic and precision medicine must be addressed to make these recommendations a reality (Box 2).
First, an effective, adaptable and sustainable model of clinical care for genomic and precision medicine is needed to address the limitations of the current genetics workforce. Second, the paucity of evidence about how best to address barriers to accessing genomic testing in Aboriginal and Torres Strait Islander people,21 culturally and linguistically diverse groups, and rural and remote communities must be considered, or we risk widening existing health care inequities and gaps in Australia. Third, upskilling non‐genetics professionals in genetics is urgently needed to enable mainstreaming of genomic medicine. Fourth, an investment in whole‐of‐system approaches, such as the Learning Healthcare Systems,22 is needed to facilitate wide‐scale education and knowledge translation at a local level. Fifth, a review of existing management and funding models for often costly advanced therapeutics is needed, with consideration for the whole patient journey, including the required genomic diagnostics and care before a patient is eligible for therapy. Finally, the introduction of genomic medicine into primary care and population screening will challenge existing health care infrastructure. Health care professions and the public need to be well equipped to understand genomics and engage in debate about ethical issues that shape our society.
A call to action: implementation science research in precision medicine
The challenges to implementing genomic and precision medicine in Australia provide an opportunity for translational research informing policy and practice. The relatively new discipline of implementation science is defined as “the scientific study of methods to promote the systematic uptake of research findings and other evidence‐based practices into routine practice, and, hence, to improve the quality and effectiveness of health services”.23 It aims to gain generalisable knowledge in a health system that could be widely applied to different providers, clinics or health systems.24 Implementation research can deal with complex health services issues more effectively than traditional clinical effectiveness research (Box 2).9
Implementation science uses theories (explaining mechanisms), models (descriptive processes) and frameworks (organisational structures and relationships)25 to plan research through stages of exploration or pre‐implementation, adoption, implementation and sustainability (Box 2).19 It focuses on identifying the barriers and facilitators (or determinants) to target and change, matching implementation strategies to these determinants, and testing strategies in real‐world settings (Box 1 and Box 2),25 as outlined in models such as the knowledge‐to‐action cycle.4 Implementation outcomes such as acceptability, adoption, sustainment and scalability are measured at individual provider, health service, and system levels.7 This structured approach can identify the mechanisms of behaviour change by selecting relevant strategies to improve evidence‐based practice adoption and adapting these for new contexts beyond the initial setting.26
An implementation science approach can address many of the already identified barriers and gaps in precision medicine (Box 1 and Box 2). Yet studies that explore these systemic adoption issues are a minority of funded genomics research. A review of genomic grants funded by the National Institutes of Health found that only 1.75% were implementation science studies.27 The Australian Genomics project28 seeks to translate genomic research into clinical practice, and is using implementation approaches and selected flagship models29 to investigate the uptake of genomics.30 The studies have identified critical factors such as a learning health care systems approach to audit and feedback, collaboration through networks, and leadership and culture in delivering genomic health care.
Other Australian research groups have demonstrated the outstanding success of genomic care in new genetic diagnoses and management pathways.31,32,33 This has led to direct implementation of genomic diagnostic testing,34 supported by state‐based funding, and further prompted strategic implementation projects; for example, the NSW Health Genomics Strategy,35 which facilitated the first TGA‐approved clinical in vivo gene therapy in Australia for retinal dystrophy36 and gene therapy for spinal muscular atrophy in newborns.
Another Australian example is the national implementation science evaluation of a mainstreaming initiative to integrate routine genetic testing for breast and ovarian cancer.37 The barriers identified can be generalised to other areas of genomic medicine, including the practitioner (role delineation) and health care system (funding and infrastructure) levels (Box 1), and identifying strategies to overcome barriers such as the use of genomic “champions”, electronic tracking systems, and defined care pathways.37
A systematic review3 of global health system interventions to embed genomic medicine into oncology identified that new models of care, interdisciplinary collaborations, and adaptable learning health systems are needed. Undertaking pre‐implementation research, which includes engagement with stakeholders, codesigning strategies, and assessment of readiness for change within organisations and the local context (or setting),38 would allow for health care planning and service delivery approaches that support and sustain equitable genomic testing adoption.3 This could make a significant difference, for example, in paediatrician‐ordered genomic sequencing in children with intellectual disability. Despite funding for paediatric genomic testing being available since 2020 and tailored educational materials (ie, implementation strategies), there has been a slow and patchy uptake.39,40 Barriers identified include a lack of time for informed genomic consent and completion of paperwork by paediatricians, which is not addressed in the funding model (Box 1).
To ensure effective models of genomic care are created, there is an urgent need for local hospital and health service and state‐based genomic medicine implementation research (Box 1). Such research would allow evidence generation for optimal adoption, knowledge of factors affecting practice, and would inform policy about precision medicine program design. A focus on pre‐implementation research commensurate with the introduction of new Medicare numbers for genomics will help define the best scalable models of care to implement genomics into routine practice. This call to action will bring the benefits of precision medicine for all Australians.
Box 1 – Opportunities, benefits, barriers and examples in the implementation of precision medicine into health care
|
|
|||||||||||||||
|
Precision medicine facilitators |
Benefits and role in precision medicine |
Barriers to adoption into standard health care |
Implementation science approaches to address barriers |
||||||||||||
|
Medicare funding for genomic tests in medicine (eg, clinical paediatrics, renal, cardiac, and other medical specialty areas) as well as state‐based funding for other conditions |
|
|
|
||||||||||||
|
Genomic oncology initiatives such as Medicare funding, germline and somatic testing strategies to inform therapy, novel targets and family risk |
|
|
|
||||||||||||
|
Advanced therapeutics such as cell and gene therapies and clinical trials (ocular; neurological, such as SMA; cancer, such as CAR‐T; and many more in future) |
|
|
|
||||||||||||
|
Population screening (eg, reproductive health with non‐invasive prenatal screening, pre‐conception carrier screening, pre‐implantation genetic diagnostics and newborn genomic screening) and public health sequencing (eg, pathogen genomics) |
|
|
|
||||||||||||
|
Future precision health technologies (eg, pharmacogenomics, polygenic risk profiles, direct to consumer testing, and predictive genomic testing informing lifestyle) |
|
|
|
||||||||||||
|
|
|||||||||||||||
|
CALD = culturally and linguistically diverse; CAR‐T = chimeric antigen receptor T cells; COVID‐19 = coronavirus disease 2019; MTB = multidisciplinary tumour boards; NDIS = National Disability Insurance Scheme; SMA = spinal muscular atrophy. Even though many new advances and opportunities exist in genomics and precision medicine, unlocking these benefits and overcoming potential barriers is a significant issue. Implementation science based research approaches7,8,9 are required at a local, health care and systemic level to select the best strategies to ensure that tailored interventions will overcome contextual barriers for each target environment, promoting the adoption of new practices into standard care. |
|||||||||||||||
Box 2 – Using implementation science to plan translational genomics research
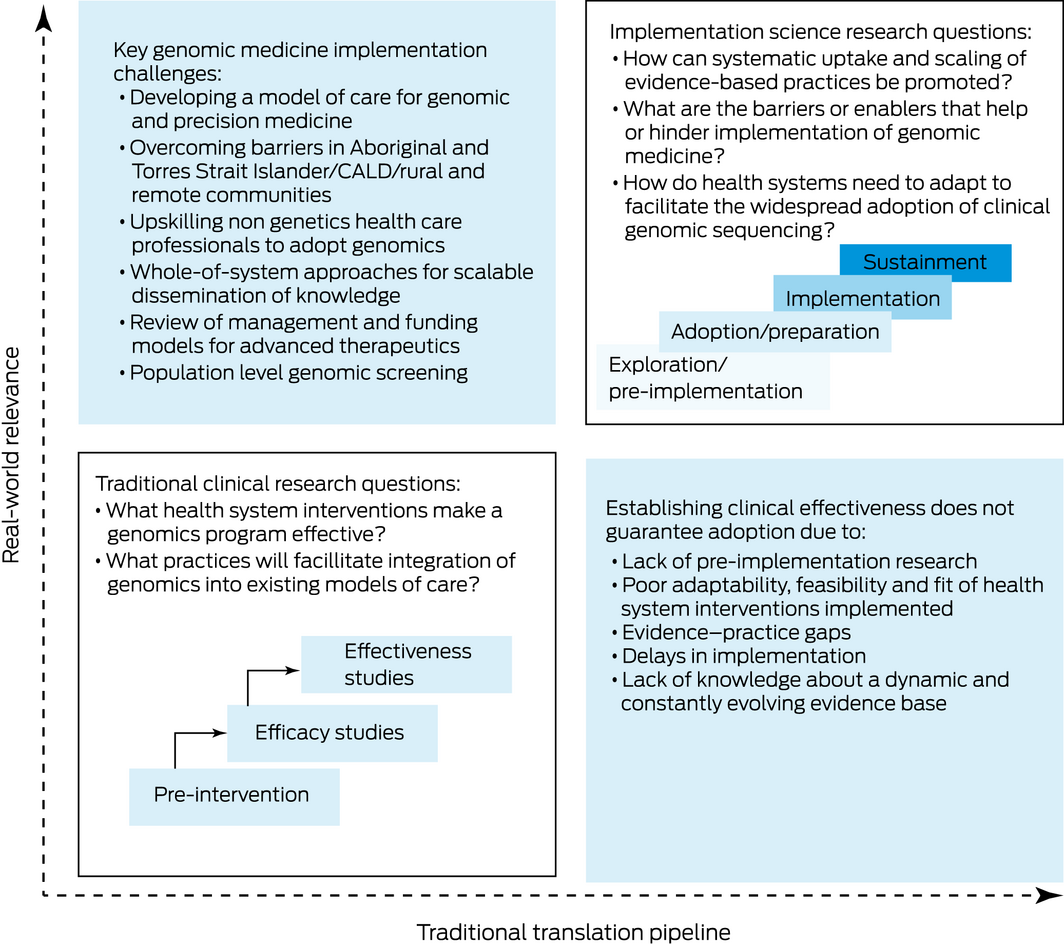
CALD = culturally and linguistically diverse. Figure adapted with permission from O'Connell et al.19 It highlights the key implementation challenges in genomic and precision medicine. It also illustrates the key differences between traditional clinical research, with its focus on intervention efficacy and effectiveness, and implementation science based research, which is focused on the “how” questions such as feasibility, sustainability and health system readiness for clinical adoption. These key differences help to address the problem that establishing clinical effectiveness alone does not guarantee clinical adoption due to many factors, such as evidence–practice gaps and localised barriers and needs.
Provenance: Not commissioned; externally peer reviewed.
- 1. Parliament of Australia. The New Frontier — delivering better health for all Australians: inquiry into approval processes for new drugs and novel medical technologies in Australia. Canberra: Commonwealth of Australia, 2021. (https://www.aph.gov.au/Parliamentary_Business/Committees/House/Health_Aged_Care_and_Sport/Newdrugs/Report (viewed June 2022).
- 2. Horowitz CR, Orlando LA, Slavotinek AM, et al. The genomic medicine integrative research framework: a conceptual framework for conducting genomic medicine research. Am J Hum Genet 2019; 104: 1088‐1096.
- 3. O'Shea R, Taylor N, Crook A, et al. Health system interventions to integrate genetic testing in routine oncology services: A systematic review. PLoS One 2021; 16: e0250379.
- 4. Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof 2006; 26:13‐24.
- 5. Jones LK, Strande NT, Calvo EM, et al. A RE‐AIM framework analysis of DNA‐based population screening: using implementation science to translate research into practice in a healthcare system. Front Genet 2022; 13: 883073.
- 6. Rigter T, Jansen ME, de Groot JM, et al. Implementation of pharmacogenetics in primary care: a multi‐stakeholder perspective. Front Genet 2020; 11: 10.
- 7. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011; 38: 65‐76.
- 8. Pinnock H, Barwick M, Carpenter CR, et al. Standards for Reporting Implementation Studies (StaRI) statement. BMJ 2017; 356: i6795.
- 9. Rankin NM, Butow PN, Hack TF, et al. An implementation science primer for psycho‐oncology: translating robust evidence into practice. J Psychosoc Oncol Res Pract 2019; 1: 14.
- 10. Kirk EP, Ong R, Boggs K, et al. Gene selection for the Australian Reproductive Genetic Carrier Screening Project (“Mackenzie's Mission”). Eur J Hum Genet 2021; 29: 79‐87.
- 11. Lacaze PA, Tiller J, Winship I; DNA Screen Investigator Group. Population DNA screening for medically actionable disease risk in adults. Med J Aust 2022; 216: 278‐280. https://www.mja.com.au/journal/2022/216/6/population‐dna‐screening‐medically‐actionable‐disease‐risk‐adults
- 12. Long JC, Gul H, McPherson E, et al. A dynamic systems view of clinical genomics: a rich picture of the landscape in Australia using a complexity science lens. BMC Med Genomics 2021; 14: 63.
- 13. Burns BL, Bilkey GA, Coles EP, et al. Healthcare system priorities for successful integration of genomics: an Australian focus. Front Public Health 2019; 7: 41.
- 14. Royal Australian and New Zealand College of Ophthalmologists. Guidelines for the assessment and management of patients with inherited retinal degenerations (IRD). RANZCO, 2020. https://ranzco.edu/policies_and_guideli/guidelines‐for‐the‐assessment‐and‐management‐of‐patients‐with‐inherited‐retinal‐degenerations‐ird/ (viewed June 2022).
- 15. Nisselle A, Macciocca I, McKenzie F, et al. Readiness of clinical genetic healthcare professionals to provide genomic medicine: an Australian census. J Genet Couns 2019; 28: 367‐377.
- 16. Clinical Genetics Network. Organisational model of care: clinical genomics model of care. Sydney: NSW Health Agency for Clinical Innovation, 2021. https://aci.health.nsw.gov.au/__data/assets/pdf_file/0006/669660/ACI‐Clinical‐genomics‐model‐of‐care.pdf (viewed June 2022).
- 17. Dragojlovic N, Borle K, Kopac N, et al. Correction: the composition and capacity of the clinical genetics workforce in high‐income countries: a scoping review. Genet Med 2020; 22: 1570.
- 18. Manolio TA. Implementing genomics and pharmacogenomics in the clinic: The National Human Genome Research Institute's genomic medicine portfolio. Atherosclerosis 2016; 253: 225‐236.
- 19. National Research Council, Institute of Medicine; O'Connell ME, Boat T, Warner KE; editors. Preventing mental, emotional, and behavioral disorders among young people: progress and possibilities. Washington, DC: National Academies Press, 2009; p 592.
- 20. Hamilton AB, Oishi S, Yano EM, et al. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med 2014; 16: 238‐245.
- 21. Baynam G, Molster C, Bauskis A, et al. Indigenous genetics and rare diseases: harmony, diversity and equity. Adv Exp Med Biol 2017; 1031: 511‐520.
- 22. Institute of Medicine (US) Roundtable on Evidence‐Based Medicine; Olsen LA, Aisner D, McGinnis JM; editors. The Learning Healthcare System: workshop summary. Washington, DC: National Academies Press, 2007.
- 23. Eccles MP, Mittman BS. Welcome to Implementation Science. Implement Sci 2006; 1: 1.
- 24. Brown CH, Curran G, Palinkas LA, et al. An overview of research and evaluation designs for dissemination and implementation. Annu Rev Public Health 2017; 38: 1‐22.
- 25. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015; 10: 53.
- 26. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015; 10: 21.
- 27. Roberts MC, Clyne M, Kennedy AE, et al. The current state of funded NIH grants in implementation science in genomic medicine: a portfolio analysis. Genet Med 2019; 21: 1218‐1223.
- 28. Stark Z, Boughtwood T, Phillips P, et al. Australian Genomics: a federated model for integrating genomics into healthcare. Am J Hum Genet 2019; 105: 7‐14.
- 29. Best S, Brown H, Lunke S, et al. Learning from scaling up ultra‐rapid genomic testing for critically ill children to a national level. NPJ Genom Med 2021; 6: 5.
- 30. Stark Z, Dolman L, Manolio TA, et al. Integrating genomics into healthcare: a global responsibility. Am J Hum Genet 2019; 104: 13‐20.
- 31. Bagnall RD, Ingles J, Dinger ME, et al. Whole genome sequencing improves outcomes of genetic testing in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2018; 72: 419‐429.
- 32. Palmer EE, Sachdev R, Macintosh R, et al. Diagnostic yield of whole genome sequencing after nondiagnostic exome sequencing or gene panel in developmental and epileptic encephalopathies. Neurology 2021; 96: e1770‐e1782.
- 33. Shih ST, Farrar MA, Wiley V, Chambers G. Newborn screening for spinal muscular atrophy with disease‐modifying therapies: a cost‐effectiveness analysis. J Neurol Neurosurg Psychiatry 2021; 92: 1296‐1304.
- 34. Ma AS, Grigg JR, Ho G, et al. Sporadic and familial congenital cataracts: mutational spectrum and new diagnoses using next‐generation sequencing. Hum Mutat 2016; 37: 371‐384.
- 35. NSW Health. NSW Health Genomics Strategy [website]. 30 June 2017 https://www.health.nsw.gov.au/services/Pages/nsw‐health‐genomics‐strategy.aspx (viewed June 2022).
- 36. Timms P, Scott S. New Australian‐first gene therapy gives Rylee and Saman sight at night — and the chance at a new life. ABC News 2021; 7 Oct. https://www.abc.net.au/news/2021‐10‐07/australian‐first‐gene‐eye‐therapy‐treatment/100518908 (viewed June 2022).
- 37. O'Shea R, Rankin NM, Kentwell M, et al. How can Australia integrate routine genetic sequencing in oncology: a qualitative study through an implementation science lens. Genet Med 2020; 22: 1507‐1516.
- 38. May CR, Johnson M, Finch T. Implementation, context and complexity. Implement Sci 2016; 11: 141.
- 39. Sachdev R, Field M, Baynam GS, et al. Paediatric genomic testing: navigating Medicare rebatable genomic testing. J Paediatr Child Health 2021; 57: 477‐483.
- 40. Shah M, Selvanathan A, Baynam G, et al. Paediatric genomic testing: navigating genomic reports for the general paediatrician. J Paediatr Child Health 2021; 58: 8‐15.






Correspondence: alan.ma@health.nsw.gov.au
Open access
Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
Alan Ma is the recipient of a Sydney Health Partners’ Research Translation Fellowship (https://sydneyhealthpartners.org.au/work‐with‐us/research‐translation‐fellowships/), which funds part of his clinical time to perform implementation science based research in genomics.
No relevant disclosures.