Future clinical trials need to focus on the steps of the pancreatic cancer continuum which have been suboptimally explored
Despite accounting for 2.8% of all new cancer cases diagnosed in Australia in 2021, pancreatic cancer contributed to 6.9% of all cancer‐related deaths.1 This is due to its poor prognosis, with 50% of patients presenting with metastatic disease at diagnosis.2 In the period 2013–2017, the 5‐year survival rate for pancreatic cancer was 11.5%, being among the lowest for all cancers and having only marginally improved from 10.7% in 2012–2016.1 Patients also experience high symptom burden across all stages of their cancer journey,3 which contributes to anxiety, depression and poor quality of life.4
The impact of pancreatic cancer combined with the urgent need to improve outcomes prompted the Australian Government to develop the National Pancreatic Cancer Roadmap.5 An important activity in informing the key priority areas of the roadmap was to undertake a review of clinical trials in pancreatic cancer to understand the gaps and opportunities in the current clinical trials landscape along the pancreatic cancer care continuum. In this perspective article, we discuss the landscape of clinical trials along the pancreatic cancer care continuum, with an emphasis on opportunities for future clinical trials.
Clinical trials across the pancreatic cancer journey
Between 1 January 2012 and 31 December 2020, 136 pancreatic cancer clinical trials (not including trials focused exclusively on pancreatic neuroendocrine tumours) were conducted in at least one Australian investigational site. Of these, 40 (29%) were investigator‐initiated and 96 (71%) were industry‐sponsored clinical trials (Box 1; Supporting Information, table 1). Of the 40 investigator‐initiated trials, 25 (63%) recruited only participants diagnosed with pancreatic cancer (ie, focused exclusively on pancreatic cancer). Of the 96 industry‐sponsored trials, 28 (29%) were focused exclusively on pancreatic cancer (Box 1).
In order to understand the key areas of focus of clinical trials, one approach is to map the interventions along the steps of the Optimal Care Pathway (OCP)6 (Box 2 and Supporting Information, table 2), which include:
- prevention and early detection;
- presentation, initial investigations, and referral;
- diagnosis, staging, and treatment planning;
- treatment (for non‐metastatic disease);
- care after initial treatment and recovery;
- management of recurrent, residual or metastatic disease; and
- end‐of‐life care, as well as care that should be provided across the care continuum, including supportive and palliative care.
Of the investigator‐initiated trials focused on pancreatic cancer (25 trials), a large proportion explored interventions along the earlier steps of the OCP, including diagnosis, staging and treatment planning (9/25, 36%) as well as treatments for non‐metastatic disease (10/25, 40%), particularly chemotherapy and/or radiotherapy for localised disease. The opposite trend was observed for industry‐sponsored trials focused on pancreatic cancer (28 trials), which predominantly investigated treatment for recurrent, residual or metastatic disease (23/28, 82%). None of the identified trials that focused exclusively on pancreatic cancer investigated prevention and early detection; presentation, initial investigations and referral; nor end‐of‐life care. Furthermore, a minimal number of investigator‐initiated trials explored supportive care‐related interventions (2/25, 8%).
In contrast, a greater proportion of investigator‐initiated trials conducted in other cancers including pancreatic cancer (15 trials) explored early detection (1/15, 7%), pharmacogenetics (1/15, 7%), prehabilitation (2/15, 13%), emerging therapies (1/15, 7%), and supportive care (5/15, 33%). Industry‐sponsored trials conducted in other cancers including pancreatic cancer (68 trials) had a greater focus on targeted therapies (20/68, 29%) and immunotherapies (16/68, 24%) in non‐metastatic disease. However, it is unclear what proportion of the overall sample in these studies would be represented by participants with pancreatic cancer.
Limited access to clinical trials in pancreatic cancer
Of the trials focused on pancreatic cancer, a large proportion of investigator‐initiated trials were single‐centre (10/25, 40%), and only four (16%) recruited internationally. In contrast, most industry‐sponsored trials (26/29, 93%) recruited internationally (Box 1). Trials mostly recruited in metropolitan areas (Box 1) across Victoria, New South Wales and South Australia. Concerningly, none of the identified trials focused on pancreatic cancer recruited in the Northern Territory (Box 3). In addition, trials did not provide information regarding the active inclusion of Indigenous or other under‐represented populations. Similar trends were observed in trials in other cancers including pancreatic cancer (Box 1).
Priority areas in pancreatic cancer
Despite delayed diagnosis,7 poor survival, and high symptom burden being notorious issues in pancreatic cancer, few clinical trials were identified in areas of prevention or early detection, initial investigations and referral, or end‐of‐life and supportive care. The Priority Setting Partnership for Pancreatic Cancer identified the question “how can patients with pancreatic cancer be offered a holistic treatment package (eg, survival time, quality of life, mobility, autonomy, among others)?” as one of its top ten research priorities.8 The limited number of trials identified in areas of palliative and supportive care indicates a significant gap in our research agenda.
Marginal improvements over time in the 5‐year survival rate for pancreatic cancer suggest that prompt investigation of novel treatment approaches is desperately needed. Our review identified that targeted therapies were predominantly investigated through trials that recruited patients with a range of cancer types. However, it could not be determined through most trial registration records what proportion of participants enrolled in these trials had pancreatic cancer and, therefore, had access to novel therapeutic approaches.
Reasons for limited clinical trials across the pancreatic cancer care continuum
There are likely numerous reasons for limited range of trials in pancreatic cancer. Difficulties recruiting participants and high attrition rates due to participants rapidly deteriorating are common challenges in the palliative care setting,9 arguably accounting for the limited number of supportive care trials in this population. A lack of clinical trials across the OCP may also be attributable to variations in funding across the cancer care continuum and may warrant targeted calls for funding.
Opportunities to broaden the landscape of clinical trials in pancreatic cancer
A prospective survey conducted in July 2015 by Cancer Australia identified that, of the overall funding allocated to cancer research in Australia, the proportion of funding committed to pancreatic cancer was low, relative to its mortality and burden of disease.10 Given that clinical trials create opportunities for breakthroughs in the early detection and treatment of cancers, targeted funding may help address the low 5‐year survival rate observed in pancreatic cancer. Future clinical trials should consider investigating interventions along key steps of the pancreatic cancer care continuum which have been neglected to date, such as prevention or early detection, initial investigations and referral, novel therapies, and end‐of‐life and supportive care‐related interventions.
Although the risk factors for pancreatic cancer are diverse and there exists no single prevention strategy, there is a strong case to be made for the investigation of early detection approaches. The development of new approaches for early detection may involve advances in imaging and/or “omics” to identify potential biomarkers, combined with big data analytics, to determine who would most benefit from improved surveillance programs.11
Supportive and palliative care trials in low survival cancer populations are often impeded by recruitment and retention challenges.12 Well designed interventions that offer flexibility and do not require lengthy and burdensome patient assessment measures may facilitate increased participation in this group of studies.13 It is possible that studies investigating supportive and palliative care or early detection techniques may be observational in nature and therefore not captured. Consequently, there is also scope for future research to investigate the landscape of observational studies in pancreatic cancer.
A greater number of national and international collaborative clinical trial programs designed by clinical investigators, such as the MoST‐P trial,14,15 which stratifies patients with pancreatic cancer by their biomarkers to receive the most promising targeted therapy, may propel efforts towards improving the quality of life and survival of patients with this type of cancer. Even though numerous challenges, including complex regulatory and ethics review requirements, high cost, and logistical issues, are common barriers faced by collaborative clinical trials,16 these barriers may be mitigated through coordinated effort and leadership from all involved stakeholders.
Access to clinical trials across different regions of Australia appeared to be disproportionate. The emerging landscape of teletrials may offer greater access to clinical trials for regional and remote participants.17 Moreover, Indigenous Australians experience significantly higher age‐standardised incidence and mortality rates of pancreatic cancer compared with non‐Indigenous Australians,18 highlighting the need for targeted recruitment of under‐represented populations. Qualitative investigations with relevant stakeholders which ensure they capture the diversity of the Australian population may assist in further understanding the current issues with conducting clinical trials in the pancreatic cancer population and identify opportunities for increased participation. Access, recruitment and retention barriers could be further mitigated by involving consumers from the outset when designing clinical trials.19
Conclusion
Overall, there is a clear urgency for future clinical trials to focus on the steps of the pancreatic cancer continuum which have been suboptimally explored to date, in areas such as early detection, initial presentation, diagnostic investigations, timely referral, novel therapies and supportive and end‐of‐life care. Targeted calls for funding for clinical trials networks may aid this process. Greater collaborative efforts by investigators located across Australian states and territories, as well as between Australia and other countries, are also warranted.
Box 1 – Characteristics of eligible clinical trials
|
|
Investigator‐initiated (n = 40) |
Industry‐sponsored (n = 96) |
|||||||||||||
|
Pancreatic cancer (exclusively) (n = 25) |
Other cancer (including pancreatic cancer) (n = 15) |
Pancreatic cancer (exclusively) (n = 28) |
Other cancer (including pancreatic cancer) (n = 68) |
||||||||||||
|
|
|||||||||||||||
|
Year of commencement* |
|
|
|
|
|||||||||||
|
pre‐2012 |
3 (12%) |
1 (7 %) |
7 (25%) |
3 (4%) |
|||||||||||
|
2012 |
3 (12%) |
0 |
2 (7%) |
3 (4%) |
|||||||||||
|
2013 |
3 (12%) |
0 |
0 |
4 (6%) |
|||||||||||
|
2014 |
0 |
0 |
5 (18%) |
1 (1%) |
|||||||||||
|
2015 |
1 (4%) |
0 |
1 (4%) |
8 (12%) |
|||||||||||
|
2016 |
0 |
1 (7 %) |
3 (11%) |
13 (19%) |
|||||||||||
|
2017 |
4 (16%) |
1 (7 %) |
3 (11%) |
10 (15%) |
|||||||||||
|
2018 |
6 (24%) |
2 (13%) |
3 (11%) |
9 (13%) |
|||||||||||
|
2019 |
4 (16%) |
3 (20%) |
2 (7%) |
7 (10%) |
|||||||||||
|
2020 |
1 (4%) |
3 (20%) |
2 (7%) |
10 (15%) |
|||||||||||
|
2021 |
0 |
3 (20%) |
0 |
0 |
|||||||||||
|
2022 |
0 |
1 (7 %) |
0 |
0 |
|||||||||||
|
Recruitment location |
|
|
|
|
|||||||||||
|
Australia only |
21 (84%) |
14 (93%) |
2 (7%) |
13 (19%) |
|||||||||||
|
International (including Australia) |
4 (16%) |
1 (7%) |
26 (93%) |
55 (81%) |
|||||||||||
|
Recruitment site |
|
|
|
|
|||||||||||
|
Single centre |
10 (40%) |
7 (47%) |
0 |
7 (10%) |
|||||||||||
|
Statewide |
5 (20%) |
5 (33%) |
0 |
2 (3%) |
|||||||||||
|
Multistate |
3 (12%) |
1 (7%) |
2 (7%) |
4 (6%) |
|||||||||||
|
International |
4 (16%) |
1 (7%) |
26 (93%) |
55 (81%) |
|||||||||||
|
Not specified |
3 (12%) |
1 (7%) |
0 |
0 |
|||||||||||
|
Recruitment region† |
|
|
|
|
|||||||||||
|
Metropolitan |
19 (76%) |
13 (87%) |
23 (82%) |
64 (94%) |
|||||||||||
|
Regional |
1 (4%) |
7 (47%) |
10 (36%) |
15 (22%) |
|||||||||||
|
Remote |
0 |
1 (7%) |
0 |
0 |
|||||||||||
|
Not specified |
6 (24%) |
1 (7%) |
5 (18%) |
4 (6%) |
|||||||||||
|
Disease stage |
|
|
|
|
|||||||||||
|
Resectable |
4 (16%) |
na |
1 (4%) |
na |
|||||||||||
|
Borderline resectable |
0 |
na |
0 |
na |
|||||||||||
|
Borderline resectable and locally advanced |
5 (20%) |
na |
0 |
na |
|||||||||||
|
Locally advanced |
4 (16%) |
na |
3 (11%) |
na |
|||||||||||
|
Locally advanced and metastatic |
3 (12%) |
na |
4 (14%) |
na |
|||||||||||
|
Metastatic |
3 (12%) |
na |
20 (71%) |
na |
|||||||||||
|
Metastatic and recurrent |
1 (4%) |
na |
0 |
na |
|||||||||||
|
Recurrent |
0 |
na |
0 |
na |
|||||||||||
|
All stages |
5 (20%) |
na |
0 |
na |
|||||||||||
|
|
|||||||||||||||
|
na = not applicable, as pancreatic cancer disease stage was not specified. * Trials that commenced before 2012 or after 2020 were eligible for inclusion if they had been registered or completed during the eligibility period (2012–2020). † Percentages do not add to 100 as a single trial may have recruited across more than one region (eg, metropolitan and regional site). |
|||||||||||||||
Box 2 – (A) Intervention focus of investigator‐initiated trials in pancreatic cancer versus trials in other cancer types (including pancreatic cancer) according to stages of the cancer trajectory in the Optimal Care Pathway (OCP). (B) Intervention focus of industry‐sponsored trials in pancreatic cancer versus trials in other cancer types (including pancreatic cancer) according to stages of the cancer trajectory in the OCP
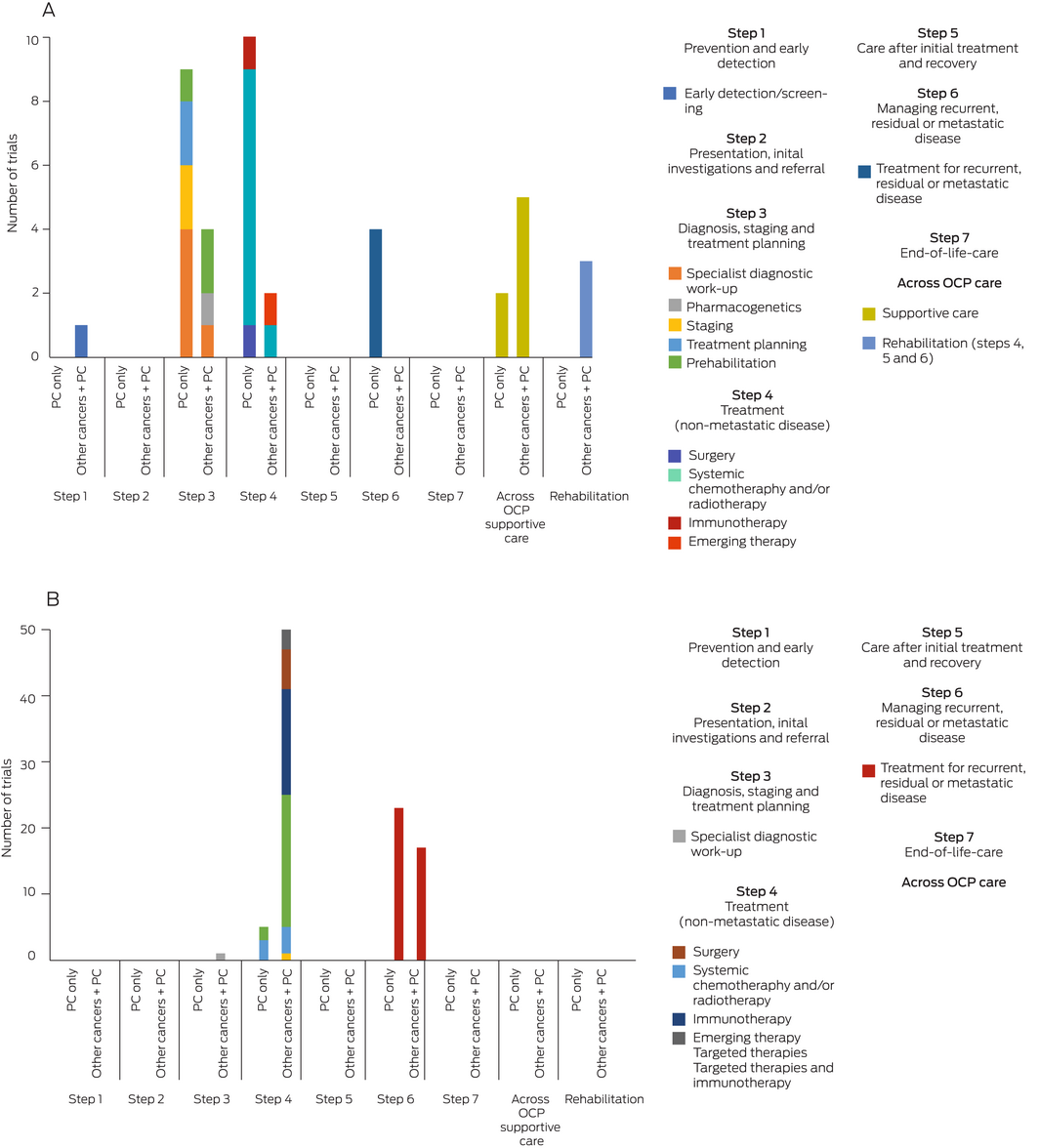
PC = pancreatic cancer.
Box 3 – Number of investigator‐initiated and industry‐sponsored trials focused exclusively on pancreatic cancer and recruiting across Australia*
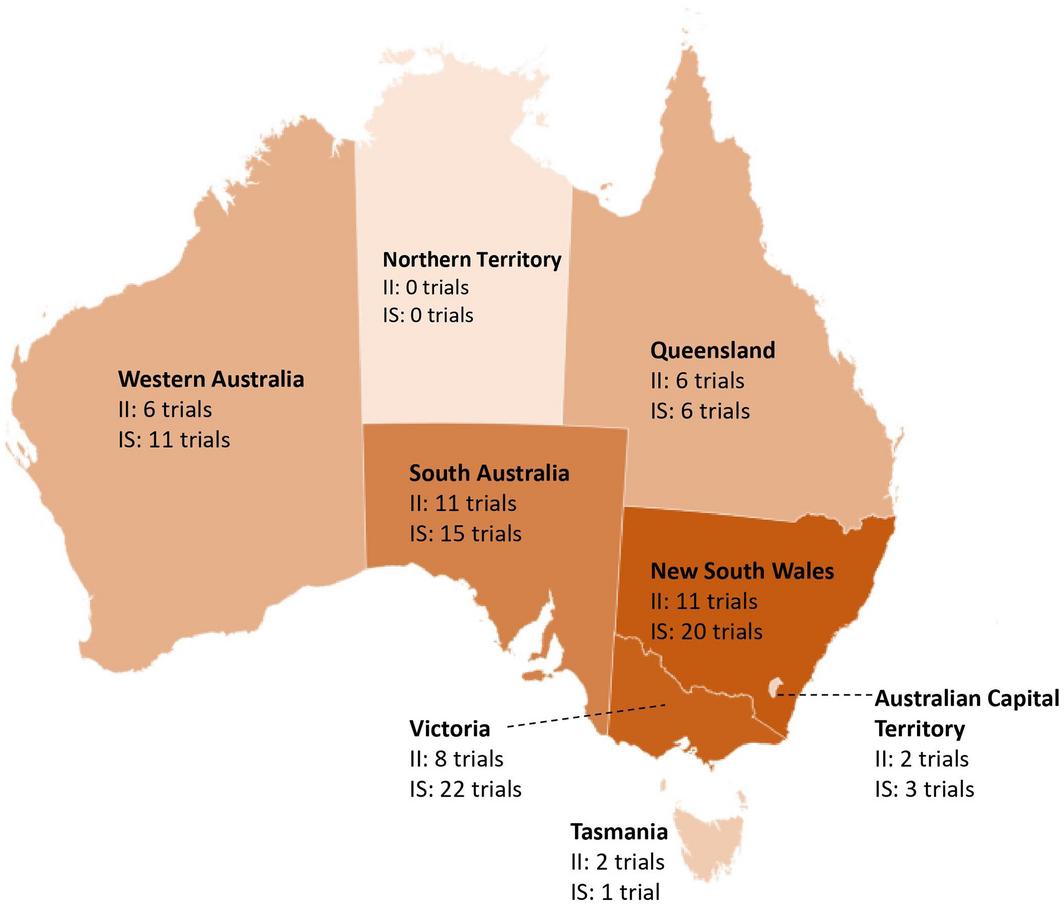
II = investigator‐initiated; IS = industry‐sponsored. * Based on data from 24 investigator‐initiated and 25 industry‐sponsored trials focused on pancreatic cancer for which recruitment location information was available. Numbers do not add up to the total number of trials as a single trial may recruit across multiple states.
Provenance: Not commissioned; externally peer reviewed.
- 1. Australian Institute of Health and Welfare. Cancer data in Australia [Cat. No. CAN 122]. https://www.aihw.gov.au/reports/cancer/cancer‐data‐in‐australia/contents/about (viewed Aug 2021).
- 2. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016; 22: 9694‐9705.
- 3. Beesley VL, Janda M, Goldstein D, et al. A tsunami of unmet needs: pancreatic and ampullary cancer patients’ supportive care needs and use of community and allied health services. Psychooncology 2016; 25: 150‐157.
- 4. Janda M, Neale RE, Klein K, et al. Anxiety, depression and quality of life in people with pancreatic cancer and their carers. Pancreatology 2017; 17: 321‐327.
- 5. Cancer Australia. National Pancreatic Cancer Roadmap [website]. https://www.canceraustralia.gov.au/key‐initiatives/national‐pancreatic‐cancer‐roadmap (viewed June 2021).
- 6. Cancer Council Victoria and Department of Health Victoria. Optimal care pathway for people with pancreatic cancer, 2nd ed; 2021. https://www.cancer.org.au/assets/pdf/pancreatic‐cancer‐optimal‐cancer‐care‐pathway#_ga=2.97729884.2133619845.1611097309‐1838291078.1605588598 (viewed Jan 2021).
- 7. Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol 2018; 24: 2047‐2060.
- 8. Klotz R, Doerr‐Harim C, Ahmed A, et al. Top ten research priorities for pancreatic cancer therapy. Lancet Oncol 2020; 21: e295‐e296.
- 9. Bouça‐Machado R, Rosário M, Alarcão J, et al. Clinical trials in palliative care: a systematic review of their methodological characteristics and of the quality of their reporting. BMC Palliative Care 2017; 16: 10.
- 10. Cancer Australia. Cancer research in Australia 2016 to 2018: opportunities for strategic research investment. https://www.canceraustralia.gov.au/sites/default/files/publications/cancer‐research‐australia‐2016‐2018‐opportunities‐strategic‐research‐investment‐summary/pdf/2016_research_review_highlights_final.pdf (viewed June 2021).
- 11. Bender E. Will a test to detect early pancreatic cancer ever be possible? Nature Outlook 2020; 579: S12‐S13.
- 12. LeBlanc TW, Lodato JE, Currow DC, Abernethy AP. Overcoming recruitment challenges in palliative care clinical trials. J Oncol Pract 2013; 9: 277‐282.
- 13. Abernethy AP, Currow DC, Wurzelmann J, et al. Enhancing enrollment in palliative care trials: key insights from a randomized, placebo‐controlled study. J Support Oncol 2010; 8: 139‐144.
- 14. Garvan Institute of Medical Research. Pancreatic cancer clinical trial program to target genome and scar tissue [media release]. 25 Feb 2021. https://www.garvan.org.au/news‐events/news/pancreatic‐cancer‐clinical‐trial‐program‐to‐target‐genome‐and‐scar‐tissue (viewed July 2021).
- 15. Thavaneswaran S, Sebastian L, Ballinger M, et al. Cancer Molecular Screening and Therapeutics (MoST): a framework for multiple, parallel signal‐seeking studies of targeted therapies for rare and neglected cancers. Med J Aust 2018; 209: 354‐345. https://www.mja.com.au/journal/2018/209/8/cancer‐molecular‐screening‐and‐therapeutics‐most‐framework‐multiple‐parallel
- 16. Tang M, Joensuu H, Simes RJ, et al. Challenges of international oncology trial collaboration — a call to action. Br J Cancer 2019; 121: 515‐521.
- 17. Collins IM, Burbury K, Underhill CR. Teletrials: implementation of a new paradigm for clinical trials. Med J Aust 2020; 213: 263‐265. https://www.mja.com.au/journal/2020/213/6/teletrials‐implementation‐new‐paradigm‐clinical‐trials
- 18. Australian Institute of Health and Welfare. Cancer in Aboriginal and Torres Strait Islander people of Australia [Cat. No. CAN 109]. https://www.aihw.gov.au/reports/cancer/cancer‐in‐indigenous‐australians (viewed Feb 2021).
- 19. Geißler J, Isham E, Hickey G, et al. Patient involvement in clinical trials. Commun Med (Lond) 2022; 2: 94.






Open access
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
This project was funded by Cancer Australia. Nadia Khan is supported by a Dora Lush (Biomedical) Research Scholarship from the Australian Government National Health and Medical Research Council (NHMRC; scholarship No. APP1191004). The contents of the published material are solely the responsibility of the individual authors and do not reflect the views of the NHMRC. We thank GlobalData for their assistance with data extraction, as well as all participants who took part in the included clinical trials.
No relevant disclosures.