Osteoarthritis affects more than 500 million people around the world, and it is a leading cause of disability.1 Non‐pharmacological strategies, such as exercise and maintaining a healthy weight, are recommended for first line management, as are simple analgesics, such as non‐steroidal anti‐inflammatory drugs (NSAIDs) and paracetamol (acetaminophen).2 However, advice on using opioid analgesics to treat the pain of knee and hip osteoarthritis is inconsistent;3,4 opioids are often prescribed, including for about 40% of people with knee osteoarthritis in the United States.5
Systematic reviews of placebo‐controlled trials of the effectiveness of opioids for treating osteoarthritis pain have been limited in scope. For example, one evaluated only opioid treatments of at least four weeks’ duration,6 while a second was restricted to oral opioid therapy.7 GRADE ratings were not always reported in the abstract or conclusions, and the validity of opioid dose–response analyses were sometimes unclear.8 One review excluded tramadol because it had been examined in a separate review.9
We therefore undertook a systematic review to provide a comprehensive evaluation of the efficacy and safety of opioid analgesic therapy regimens for people with osteoarthritis, and to explore dose–effect relationships.
Methods
We searched MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, the Allied and Complementary Medicine Database (AMED), the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials (CENTRAL) for eligible randomised controlled trials (RCTs) published in any language in peer‐reviewed journals to 31 October 2020 (Supporting Information, table 1). To avoid overestimating treatment effects,10 we also searched the World Health Organization International Clinical Trials Registry (https://trialsearch.who.int) for unpublished trials. We screened the reference lists of retrieved publications to identify further RCTs. Our systematic review was prospectively registered with PROSPERO (CRD420191142813; 16 October 2019).
Inclusion criteria
We included RCTs in which the analgesic effect of an opioid was compared with that of placebo in people with osteoarthritis of any type (knee, hip, hand, spine) of any duration. All single ingredient and combination opioid‐containing analgesic regimens were included, regardless of opioid dose and administration route. We did not include trials in which the effect of opioid therapy was compared with other treatments but not with placebo. Trials including a range of pain conditions were eligible if data for participants with osteoarthritis could be extracted.
Study selection
Three authors (CAS, WA, GZ) independently screened the titles and abstracts and read the full text of potentially eligible publications; disagreements were resolved by consensus.
Data extraction and management
Two authors from a pool of four authors (WA, GZ, CAS, SEG) independently extracted study and participant characteristics and outcomes data. They also assessed the risk of bias with the Cochrane Collaboration tool;11 each of the seven risk items were rated high, low, or unclear. Disagreements were resolved by consensus.
The major outcome of interest was pain intensity measured using a visual analogue scale, numeric rating scale, or other continuous measure. We also extracted data on disability (Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] physical function scale), health‐related quality of life (36‐item Short Form health survey [SF‐36], the European Quality of Life scale, or the Patient Generated Index), and adverse events.
Outcomes were classified as immediate (follow‐up less than two weeks after randomisation), short (two to less than six weeks), medium (six weeks to less than 12 months), or long term (12 months or more). The primary outcome for our analysis was medium term pain relief.
Continuous outcomes were converted to a 0–100 scale, and we report mean differences (MDs) in outcomes (not standardised mean differences) with 95% confidence intervals (CIs). We classified between‐group differences of 0–9 points as very small, 10–19 points as small, 20–29 points as moderate, and differences of 30 points or more as large. If authors reported both mean follow‐up and mean change scores, we report the follow‐up mean scores and standard deviations (SDs) if the baseline group scores were similar. For dichotomous outcomes, we report relative risks (RRs) with 95% CIs.
We extracted data for adverse events, and the numbers of trial participants receiving opioid therapy who withdrew during the run‐in (for enriched trial designs) or trial phases because of adverse events or lack of efficacy, or who were lost to follow‐up. Missing data were imputed using methods described in the Cochrane handbook for systematic reviews.12
We assessed trial heterogeneity (in terms of the characteristics of participants, interventions, outcome measures, and timing of outcome measurement) by visual inspection of forest plots and with the I2 statistic.
Statistical analyses were conducted in R 4.0.3 (R Foundation for Statistical Computing) and R Studio 1.2.1093 (https://www.rstudio.com/products/rstudio).
Data synthesis
We pooled outcomes data using restricted maximum likelihood estimation in random effects meta‐analysis models. Three‐level mixed effects models (fitted in R with the metafor 3.0‐1 package13) accounted for the non‐independence of effect sizes from the same study or comparator group, and assumed a compound symmetry covariance structure.
We used meta‐regression in the same three‐level models to evaluate associations of opioid dose with medium term impact on pain and adverse events. Single ingredient opioid analgesic doses were converted to oral morphine milligram equivalent (MME) doses14 and log‐transformed prior to meta‐regression.
In our primary meta‐analysis, we assessed the pooled effects of all single ingredient opioid analgesics. In a separate analysis, we calculated treatment effect sizes for combination analgesics including an opioid and a simple analgesic (paracetamol or NSAID).
Quality of evidence
We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria15 to evaluate the overall quality of evidence. Quality of evidence was downgraded one level for each of four factors:
- limitation in study design: the risk of bias was high (one or more domains were judged to be at high risk) for more than one‐quarter of studies included in an analysis;11
- inconsistency of results: statistical heterogeneity was large (I2 > 50%);16
- imprecision: confidence interval width was greater than ten points (continuous outcomes) or included zero (dichotomous outcomes); and
- publication bias: assessed by funnel plot analysis or with the Egger regression test17 (for ten or more studies).
Overall quality of evidence was classified as high, moderate, low, or very low (Supporting Information, table 2).13
Sensitivity analyses
We conducted sensitivity analyses that assessed the impact on treatment effects of opioid formulation (modified or immediate release preparations) and study design (standard or enriched), to compare the effects of tramadol with those of other opioids, and to assess the effects of industry funding, trial report type (peer‐reviewed publication or unpublished), and the use of rescue medication.
Protocol amendments after PROSPERO registration
We conducted sensitivity (rather than subgroup) analyses of the effects of formulation and study design. The tramadol sensitivity analysis was added because the medication is recommended by some guidelines for people with osteoarthritis.4 The sensitivity analyses of the effects of industry funding, trial report (peer‐reviewed publication or unpublished), and the use of rescue medication were added after PROSPERO registration.
Results
We initially identified 2286 relevant publications, of which 74 were deemed potentially eligible after abstract review; 38 were excluded after reviewing the full text, and 36 publications (all in English) were included in our analysis18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53 (Box 1; Supporting Information, tables 3 and 4).
Study participants
The eligible trials included people with high pain levels; the median score at baseline for all included trials was 72.0 points (interquartile range [IQR], 61.7–75.8 points). A median of 42% of participants in all opioid trial arms (IQR, 33–52%) and of 40% of participants in the control arms (IQR, 24–48%) discontinued participation during trial phases. The most frequent reasons for withdrawal from the opioid arms were adverse events (median, 24% of participants; IQR, 15–30%), lack of efficacy (median, 7.8%; IQR, 3.8–11%), and other reasons (loss to follow‐up, protocol violation: median, 6.8%; IQR, 3.6–12%) (Supporting Information, table 5 and figure 1).
Treatment regimens
The included studies evaluated oral or transdermal opioid analgesic medications (modified release formulations) for people with osteoarthritis, chiefly of the knee, hip, or both. The opioid analgesics evaluated were tramadol, oxycodone, tapentadol, hydromorphone, codeine, buprenorphine, fentanyl, oxymorphone, tramadol/acetaminophen, oxycodone/acetaminophen, and ibuprofen/codeine; five studies evaluated transdermal buprenorphine or fentanyl.34,35,36,43,45 Doses ranged from 10 to 210 MME per day, treatment duration from one day (200 mg ibuprofen/30 mg codeine, for hip osteoarthritis) to 12 weeks; 15 studies18,19,20,21,22,24,25,28,29,31,33,35,39,41,45 reported treatment durations of four weeks or less, with regular (ie, not “when required”) regimens (Supporting Information, tables 3 and 6).
Trial characteristics
For all but two studies25,28 the risk of bias was rated as high (Supporting Information, table 7). The longest follow‐up period was 16 weeks;51 19 trials had follow‐up periods of 12 weeks.23,26,27,30,32,36,37,38,40,42,43,44,46,47,48,49,50,51,53 Treatment duration was usually the same length as the follow‐up period (Supporting Information, table 3). Four trials36,37,44,47 had enrichment trial designs, whereby patients who completed an open label run‐in phase during which they tolerated and responded to the opioid medication were included in the trial phase.
Treatment effects: pain
For the primary outcome (medium term pain relief), the evidence from 19 trials23,26,30,32,34,36,37,38,40,42,43,44,46,48,49,50,51,52,53 (8965 patients; dose range, 10–126 MME/day) for a very small effect (MD, –4.59 points; 95% CI, –7.17 to –2.02 points) was low quality (Box 2; Supporting Information, table 8). There was moderate quality evidence from 13 trials19,21,22,24,26,28,29,32,34,42,48,50,53 (5320 patients; dose range, 10–126 MME/day) for a very small immediate effect (MD, –4.90 points; 95% CI, –6.46 to –3.34 points), and from 19 trials20,21,22,24,26,28,29,30,31,32,33,34,35,41,42,45,48,50,53 (6949 patients; dose range, 10–210 MME/day) for a very small short term effect (MD, –6.38 points; 95% CI, –8.45 to –4.30 points) (Supporting Information, tables 8 and 9; figures 2 and 3).
Four studies18,20,25,27 compared the effects of combinations of opioids and simple analgesics with that of placebo. Moderate quality evidence suggested that 6 × 200 mg ibuprofen/30 mg codeine per day has a small effect in the immediate term on hip osteoarthritis pain (MD, −19.0 points; 95% CI, −31.2 to −6.8 points),18 and low quality evidence that tramadol/acetaminophen provides very small pain relief in the immediate25 and medium terms.25,27 Low quality evidence from one trial20 suggested that 4 × 5 mg oxycodone (immediate release)/325 mg acetaminophen per day provides small short term pain relief (MD, –17.0 points; 95% CI, –30.3 to –3.72 points).
Treatment effects: disability
Evidence from 16 trials23,26,30,32,34,38,40,42,43,44,46,48,49,50,51,53 (6882 patients; dose range, 10–126 MME/day) for a very small medium term effect on disability as assessed with the WOMAC physical function scale (MD, –4.15 points; 95% CI, –6.94 to –1.35 points) was low quality. There was moderate quality evidence from three trials24,32,46 (2105 patients; dose range, 10–40 MME/day) for a very small immediate effect (MD, –4.11 points; 95% CI, –6.92 to –1.30 points), and from eight trials21,24,30,31,32,33,46,50 (3394 patients; dose range 10–210 MME/day) for a very small short term effect (MD, –5.84 points; 95% CI, –7.90 to –3.79 points) (Box 3; Supporting Information, tables 8 and 10). The effect of opioids on WOMAC total score was very small at each time point (Supporting Information, tables 8 and 11).
Treatment effects: quality of life
Opioids had no statistically significant effect on mean SF‐36 mental component scores in the short and medium terms. They had a very small effect on the SF‐36 physical component score in the short term (two trials;31,33 824 patients; MD, 3.05 points; 95% CI, 1.13–4.97 points) and medium term (five trials;32,34,42,46,50 3525 patients; MD, 0.70 points, 95% CI, 0.04–1.37 points). Only one study30 (107 patients) found a favourable medium term effect of controlled‐release oxycodone on quality of life (76 MME/day) measured with the Patient Generated Index (MD, 11.5 points; 95% CI, 2.37–20.6 points) (Supporting Information, tables 8 and 12).
Adverse events
There was very low quality evidence from 16 studies26,30,32,34,36,38,40,42,43,44,46,51,53 (8482 patients) that opioids (dose range, 10–126 MME/day) increased the risk of adverse events at medium term follow‐up (v placebo: RR, 1.43; 95% CI, 1.29–1.59). In these 16 studies, 3871 of 5349 people in the opioid arms (72.4%) and 1560 of 3133 in the control arms (49.8%) had experienced adverse events, most frequently gastrointestinal events (nausea, vomiting, constipation, diarrhoea); other common side effects were dry mouth, fatigue, pruritus, somnolence, dizziness, and headache (Supporting Information, tables 8, 13, 14; figure 4). In five trials that compared tramadol with placebo,26,32,38,40,46 1493 of 2192 people in the tramadol arms (68.1%) and 469 of 981 in the placebo arms (47.8%) reported adverse events (RR, 1.34; 95% CI, 1.22–1.48).
Meta‐regression analyses
In meta‐regression analyses, the associations of log(MME dose) with pain relief (19 trials;23,26,30,32,34,36,37,38,40,42,43,44,46,48,49,50,51,52,53 8965 patients; regression coefficient, 0.92; 95% CI, –6.58 to 8.41) and adverse events at medium term follow‐up (16 trials26,30,32,34,36,38,40,42,43,44,46,51,53; 8482 patients; regression coefficient, 0.08; 95% CI, –0.17 to 0.33) were not statistically significant (Box 4; Supporting Information, table 8). The evidence for these findings was of very low quality.
Sensitivity analyses
Sensitivity analyses indicated that the medium term effects of tramadol on pain (–8.13 points; 95% CI, –11.8 to –4.51 points) and disability (–7.44 points; 95% CI, –12.7 to –2.16 points respectively) were statistically significant, as were the medium term effects of immediate release preparations on pain (–9.50 points; 95% CI, –19.0 to –0.04 points ); the effects, however, were very small (Supporting Information, table 15).
Discussion
We found moderate to low quality evidence that opioid medications have very small effects on pain and disability in people with osteoarthritis up to three months after the initiation of therapy. The incidence of adverse events, including gastrointestinal and central nervous system effects, was higher for people receiving opioids than for those receiving placebo, but evidence that opioids increase the risk of adverse events in the medium term was of very low quality. Meta‐regression did not indicate a significant association between opioid medication dose (range, 10–126 MME/day) and pain relief or the incidence of adverse events at medium term follow‐up.
Our review is the first systematic review of therapy for people with osteoarthritis to conclude that single ingredient opioid medications provide very small immediate, short, and medium term benefits, and that opioid dose (in the range examined) may not influence pain relief in the medium term. This is in contrast to conventional thinking that higher doses are more beneficial, and our findings should discourage the prescribing of stronger opioid analgesics (ie, higher MME equivalence) for people with severe osteoarthritis pain. Given their very small effects, the appropriateness of single ingredient opioids for managing osteoarthritis is debatable. Our findings also indicate that the association between opioid dose and medium term risk of adverse events is unclear.
However, combinations of low dose opioids with simple analgesics may have beneficial synergistic effects. For example, the 95% confidence intervals for the pain relief achieved by low dose codeine with ibuprofen and low dose oxycodone with acetaminophen included large effects (more than 30 points). For some people with osteoarthritis, short term use of these combination analgesics may be a reasonable option; however, this finding was based on a small number of studies.
The pattern of prescribing during the first month of opioid therapy is critical for the future risk of persistent use, which is particularly high for long‐acting opioid preparations.54 As many as 25% of people with osteoarthritis who commence opioid analgesic therapy are still taking them one year later.55 Another problem is that osteoarthritis, as a chronic disease, is often treated with modified release opioid preparations; 26 studies included in our review evaluated such preparations. Modified release preparations are taken regularly to moderate the variation between peak and trough levels, providing more consistent pain relief. As they are typically taken “regularly to control pain”56 rather than as required, it can be very difficult for patients to stop using them.54
Guideline recommendations regarding the use of opioids for treating osteoarthritis pain are inconsistent. Our findings indicate that opioids provide pain relief similar to that of paracetamol,57 and their benefit is almost half that achieved by NSAIDs (a conclusion, however, based on indirect comparisons),58 challenging beliefs that simpler analgesics are less effective than opioids for people with common musculoskeletal conditions.
Strengths and weaknesses
Our systematic review is the largest and most comprehensive of opioid therapy for osteoarthritis pain. The most recent review of this topic, for instance, included only 18 trials.7 Earlier reviews have excluded tramadol studies,9 evaluated only oral preparations,8 or included only treatment regimens of more than one month.6 Further, review authors have generally reported outcomes as standardised mean differences (ie, proportions of standard deviations), whereas we present mean differences on a common 0–100 pain scale to facilitate easier interpretation by clinicians and patients.
However, study heterogeneity was high for the assessments of some effects. Opioid regimens ranged from one day to 12 weeks in duration, and dosage from 10 to 210 MME/day. Further, risk of bias was high for all but two trials,25,28 and some trials had enrichment design that may have led to more optimistic effect estimates, as only participants who responded to and tolerated the medicine during the run‐in phase entered the main trial phase. Enrichment trial designs and high participant withdrawal rates during main trial phases meant that our effect estimates are based upon outcomes for about half of the participants who entered these trials. Finally, most of the included studies were undertaken in the United States, and country‐specific ethnic, cultural, or health service features may have influenced outcomes.
Conclusion
Opioid medications may provide people with osteoarthritis very small benefits but also may increase the risk of adverse events. The association between opioid dose, pain relief, and risk of adverse events requires further evaluation. Alternative pain management strategies for people with osteoarthritis should be investigated, as well as opioid‐sparing and tapering strategies for those being treated with opioids.
Box 1 – Selection of publications for inclusion in our analysis
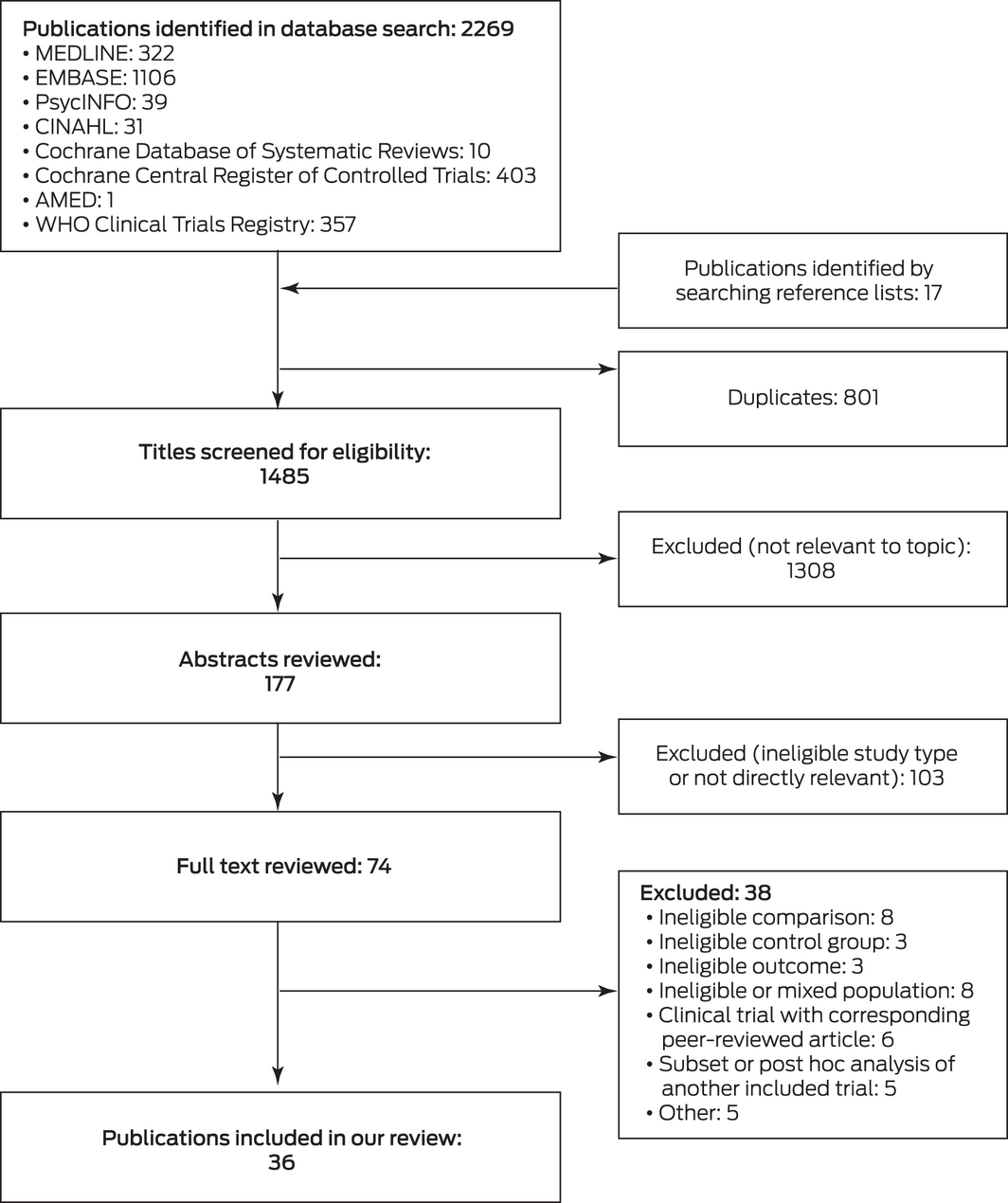
AMED = Allied and Complementary Medicine Database; CINAHL = Cumulative Index to Nursing and Allied Health Literature. ♦
Box 2 – Medium term effects of opioid medications on pain in people with osteoarthritis pain: estimated mean differences (MDs) with 95% confidence intervals (CIs)*
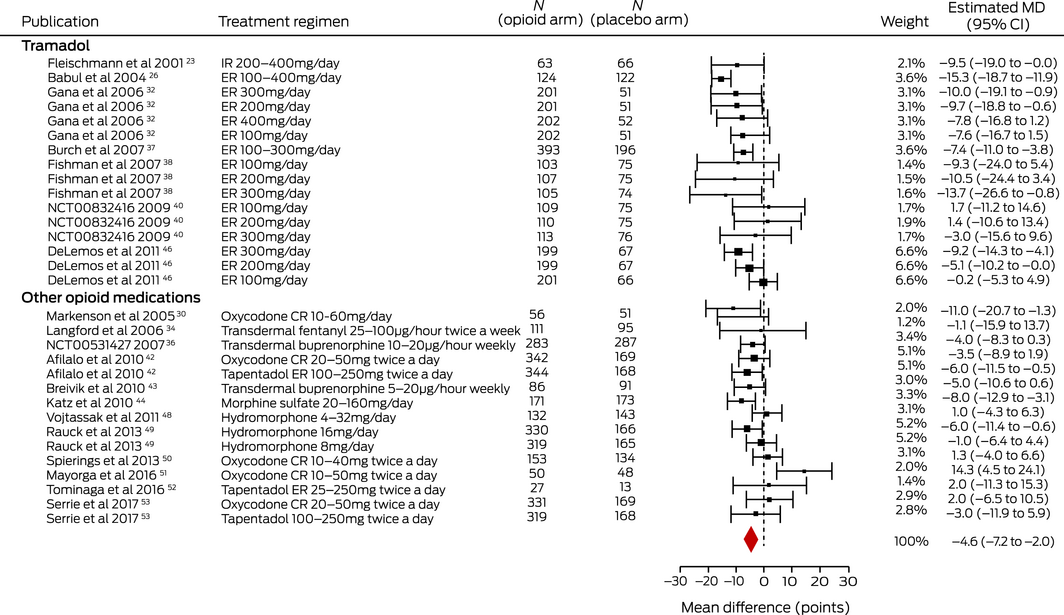
CR = controlled release; ER = extended release; IR = immediate release. * I2 = 69%. ♦
Box 3 – Medium term effects of opioid medications on disability in people with osteoarthritis: estimated mean differences (MDs) with 95% confidence intervals (CIs)*
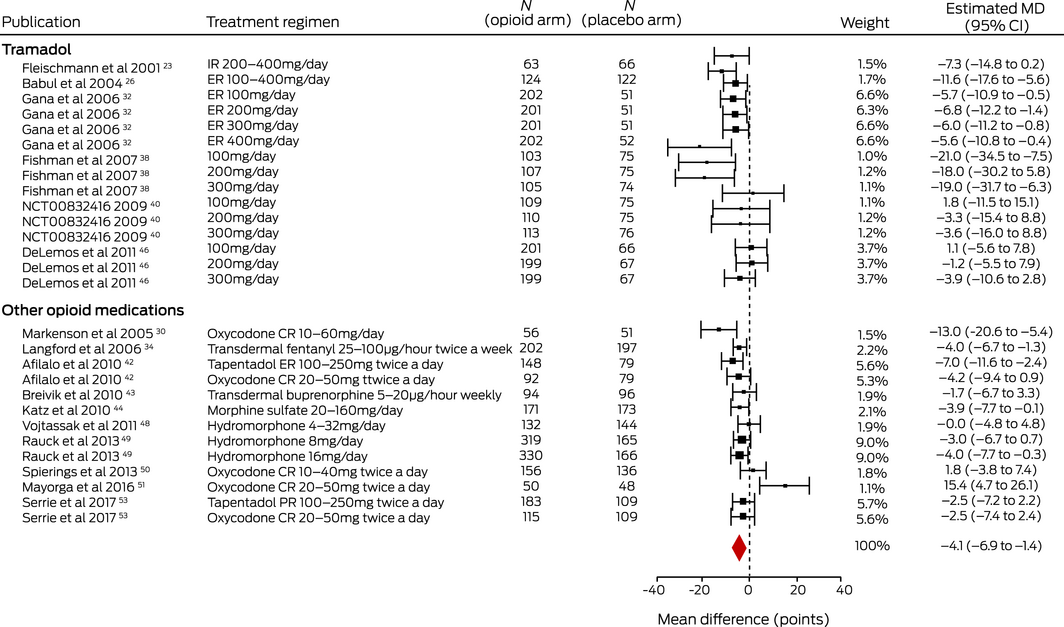
CR = controlled release; ER = extended release; IR = immediate release; PR = prolonged release. * I2 = 76%. ♦
Box 4 – Meta‐regression of the relationships between the log‐transformed morphine milligram equivalent (MME) dose and medium term pain treatment effect (A) and adverse events (B)*
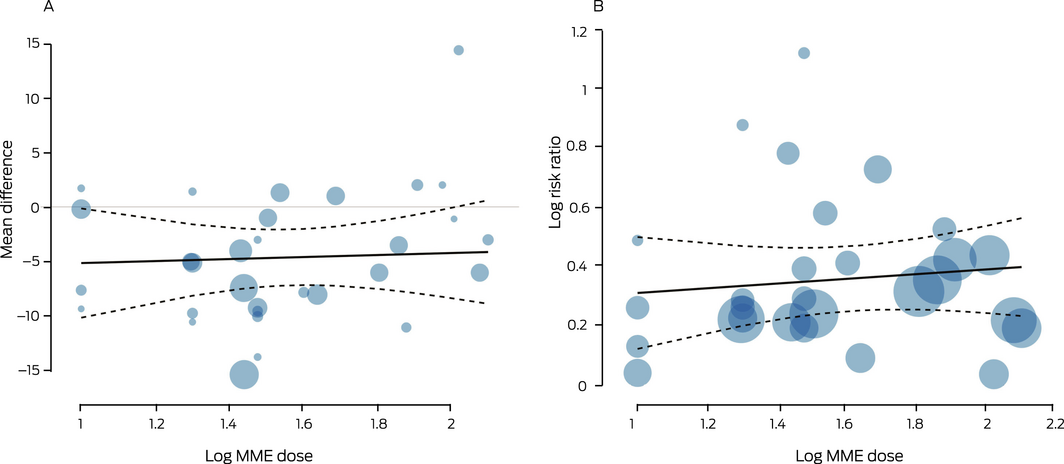
*Each circle represents an eligible comparison in the included publications (the number of comparisons exceeded the total number of publications for each outcome); circle size is proportional to sample size. The dashed lines encloses the 95% confidence region. The regression equations are: Y = –6.0 + 0.92*log(MME dose) (A); Y = 0.23 + 0.08*log(MME dose) (B). ♦
Received 15 January 2021, accepted 17 August 2021
- Christina Abdel Shaheed1
- Wasim Awal2
- Geoffrey Zhang3
- Stephen E Gilbert4
- Daniel Gallacher5
- Andrew McLachlan1,6
- Richard O Day7,8
- Giovanni E Ferreira4
- Caitlin MP Jones4
- Harbeer Ahedi4
- Mamata Tamrakar4
- Fiona M Blyth6
- Fiona Stanaway1
- Christopher G Maher1,4
- 1 The University of Sydney, Sydney, NSW
- 2 Griffith University, Gold Coast, QLD
- 3 Royal Prince Alfred Hospital, Sydney, NSW
- 4 Institute for Musculoskeletal Health, University of Sydney and Sydney Local Health District, Sydney, NSW
- 5 University of Warwick, Coventry, United Kingdom
- 6 Centre for Education and Research on Ageing, University of Sydney, Sydney, NSW
- 7 St Vincent's Hospital, Sydney, NSW
- 8 St Vincent's Clinical School UNSW, Sydney, NSW
Wasim Awal received a University of Sydney Summer Scholarship to undertake this review. Christina Abdel Shaheed is supported by a University of Sydney early career development fellowship. Christopher Maher is supported by a National Health and Medical Research Council (NHMRC) Investigator Fellowship.
Christopher G Maher, Richard O Day, and Andrew J McLachlan were investigators in the PACE study (paracetamol for acute lower back pain; ACTN 12609000966291), funded by the NHMRC and GlaxoSmithKline Australia. The Sydney Pharmacy School receives research funding from GlaxoSmithKline Australia for a research student supervised by Andrew McLachlan. Flexeze provided heat wraps at no cost to Christopher G Maher and Christina Abdel Shaheed for a feasibility trial and an investigator‐initiated trial.
- 1. Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet 2020; 396: 1711–1712.
- 2. Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the US bone and joint initiative. Semin Arthritis Rheum 2014; 43: 701–712.
- 3. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020; 72: 149–162.
- 4. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence‐based guideline. J Am Acad Orthop Surg 2013; 21: 571–576.
- 5. Huizinga JSE, Song S, Sullivan J, et al. Direct medical and societal cost of opioid use in symptomatic knee osteoarthritis patients in the United States [abstract]. Arthritis Rheumatol 2019; 71 (Suppl 10): abstract 1129.
- 6. Welsch P, Petzke F, Klose P, Häuser W. Opioids for chronic osteoarthritis pain: an updated systematic review and meta‐analysis of efficacy, tolerability and safety in randomized placebo‐controlled studies of at least 4 weeks double‐blind duration. Eur J Pain 2020; 24: 685–703.
- 7. Osani MC, Lohmander LS, Bannuru RR. Is there any role for opioids in the management of knee and hip osteoarthritis? A systematic review and meta‐analysis. Arthritis Care Res (Hoboken) 2021; 73: 1413–1424.
- 8. Lötsch J. Pharmacokinetic–pharmacodynamic modeling of opioids. J Pain Symptom Manage 2005; 29 (5 Suppl): S90–S103.
- 9. da Costa BR, Nüesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev 2014; CD003115.
- 10. Bagg MK, O’Hagan E, Zahara P, et al. Systematic reviews that include only published data may overestimate the effectiveness of analgesic medicines for low back pain: a systematic review and meta‐analysis. J Clin Epidemiol 2020; 124: 149–159.
- 11. Cochrane Collaboration. The Cochrane Collaboration’s tool for assessing risk of bias. In: Cochrane handbook for systematic reviews of interventions, version 5.10. Updated Mar 2011. https://handbook‐5‐1.cochrane.org/chapter_8/table_8_5_a_the_cochrane_collaborations_tool_for_assessing.htm (viewed Dec 2019).
- 12. Cochrane Collaboration. General principles for dealing with missing data. In: Cochrane Handbook for systematic reviews of interventions, version 5.10. Updated Mar 2011. https://handbook‐5‐1.cochrane.org/chapter_16/16_1_2_general_principles_for_dealing_with_missing_data.htm (viewed Dec 2019).
- 13. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48.
- 14. National Center for Injury Prevention and Control. CDC Compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors. Sept 2018. Archived: https://web.archive.org/web/20190618113958if_/https://www.cdc.gov/drugoverdose/data‐files/CDC_Oral_Morphine_Milligram_Equivalents_Sept_2018.xlsx (viewed Oct 2020).
- 15. Atkins D, Best D, Briss PA, et al; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490.
- 16. Guyatt GH, Oxman AD, Kunz R, et al; GRADE Working Group. GRADE guidelines. Rating the quality of evidence–inconsistency. J Clin Epidemiol 2011; 64: 1294–1302.
- 17. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
- 18. Quiding H, Grimstad J, Rusten K, et al. Ibuprofen plus codeine, ibuprofen, and placebo in a single‐ and multidose cross‐over comparison for coxarthrosis pain. Pain 1992; 50: 303–307.
- 19. Roth SH. Efficacy and safety of tramadol HCl in breakthrough musculoskeletal pain attributed to osteoarthritis. J Rheumatol 1998; 25: 1358–1363.
- 20. Caldwell JR, Hale ME, Boyd RE, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: a double blind, randomized, multicenter, placebo controlled trial. J Rheumatol 1999; 26: 862–869.
- 21. Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. J Rheumatol 2000; 27: 764–771.
- 22. Roth SH, Fleischmann RM, Burch FX, et al. Around‐the‐clock, controlled‐release oxycodone therapy for osteoarthritis‐related pain: placebo‐controlled trial and long‐term evaluation. Arch Intern Med 2000; 160: 853–860.
- 23. Fleischmann RM, Caldwell JR, Roth SH, et al. Tramadol for the treatment of joint pain associated with osteoarthritis: a randomized, double‐blind, placebo‐controlled trial. Curr Ther Res 2001; 62: 113–128.
- 24. Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once‐daily morphine formulation in chronic, moderate‐to‐severe osteoarthritis pain: results from a randomized, placebo‐controlled, double‐blind trial and an open‐label extension trial. J Pain Symptom Manage 2002; 23: 278–291.
- 25. Silverfield JC, Kamin M, Wu SC, Rosenthal N; CAPSS‐105 Study Group. Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: a multicenter, outpatient, randomized, double‐blind, placebo‐controlled, parallel‐group, add‐on study. Clin Ther 2002; 24: 282–297.
- 26. Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended‐release, once‐daily tramadol in chronic pain: a randomized 12‐week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage 2004; 28: 59–71.
- 27. Emkey R, Rosenthal N, Wu SC, et al; CAPSS‐114 Study Group. Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add‐on therapy for osteoarthritis pain in subjects receiving a COX‐2 nonsteroidal antiinflammatory drug: a multicenter, randomized, double‐blind, placebo‐controlled trial. Journal Rheumatol 2004; 31: 150–156.
- 28. Malonne H, Coffiner M, Sonet B, et al. Efficacy and tolerability of sustained‐release tramadol in the treatment of symptomatic osteoarthritis of the hip or knee: a multicenter, randomized, double‐blind, placebo‐controlled study. Clin Ther 2004; 26: 1774–1782.
- 29. Chindalore VL, Craven RA, Yu KP, et al. Adding ultralow‐dose naltrexone to oxycodone enhances and prolongs analgesia: a randomized, controlled trial of Oxytrex. J Pain 2005; 6: 392–399.
- 30. Markenson JA, Croft J, Zhang PG, Richards P. Treatment of persistent pain associated with osteoarthritis with controlled‐release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain 2005; 21: 524–535.
- 31. Matsumoto AK, Babul N, Ahdieh H. Oxymorphone extended‐release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: results of a randomized, double‐blind, placebo‐ and active‐controlled phase III trial. Pain Med 2005; 6: 357–366.
- 32. Gana TJ, Pascual ML, Fleming RR, et al; 023 Study Group. Extended‐release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double‐blind, placebo‐controlled clinical trial. Curr Med Res Opin 2006; 22: 1391–1401.
- 33. Kivitz A, Ma C, Ahdieh H, Galer BS. A 2‐week, multicenter, randomized, double‐blind, placebo‐controlled, dose‐ranging, phase III trial comparing the efficacy of oxymorphone extended release and placebo in adults with pain associated with osteoarthritis of the hip or knee. Clin Ther 2006; 28: 352–364.
- 34. Langford R, McKenna F, Ratcliffe S, et al. Transdermal fentanyl for improvement of pain and functioning in osteoarthritis: a randomized, placebo‐controlled trial. Arthritis Rheum 2006; 54: 1829–1837.
- 35. Purdue Pharma LP. Safety and efficacy of buprenorphine transdermal system in subjects with moderate to severe osteoarthritis of hip or knee (ClinicalTrials.gov Identifier: NCT00313846). Apr 2006; updated Sept 2012. https://clinicaltrials.gov/ct2/show/NCT00313846 (viewed Dec 2019).
- 36. Purdue Pharma LP. Buprenorphine transdermal system (BTDS) in subjects w/mod‐sev osteoarthritis (OA) chronic pain of knee (ClinicalTrials.gov Identifier: NCT00531427). Sept 2007; updated Sept 2012. https://clinicaltrials.gov/ct2/show/study/NCT00531427 (viewed Dec 2019).
- 37. Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage 2007; 34: 328–338.
- 38. Fishman RL, Kistler CJ, Ellerbusch MT, et al. Efficacy and safety of 12 weeks of osteoarthritic pain therapy with once‐daily tramadol (Tramadol Contramid OAD). J Opioid Manag 2007; 3: 273–280.
- 39. Hartrick C, Van Hove I, Stegmann JU, et al. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end‐stage joint disease: a 10‐day, phase III, randomized, double‐blind, active‐ and placebo‐controlled study. Clin Ther 2009; 31: 260–271.
- 40. Labopharm Inc. A four‐arm study comparing the analgesic efficacy and safety of tramadol once a day 100, 200 and 300 mg versus placebo for the treatment of pain due to osteoarthritis of the knee (ClinicalTrials.gov Identifier: NCT00832416). Jan 2009; updated Apr 2012. https://clinicaltrials.gov/ct2/show/NCT00832416 (viewed Dec 2019).
- 41. Cubist Pharmaceuticals LLC. Efficacy and safety study evaluating ADL5859 and ADL5747 in participants with pain due to osteoarthritis of the knee (ClinicalTrials.gov Identifier: NCT00979953). Sept 2009; updated Aug 2015. https://clinicaltrials.gov/ct2/show/NCT00979953 (viewed Dec 2019).
- 42. Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double‐blind, placebo‐ and active‐controlled phase III study. Clin Drug Investig 2010; 30: 489–505.
- 43. Breivik H, Ljosaa TM, Stengaard‐Pedersen K, et al. A 6‐months, randomised, placebo‐controlled evaluation of efficacy and tolerability of a low‐dose 7‐day buprenorphine transdermal patch in osteoarthritis patients naive to potent opioids. Scand J Pain 2010; 1: 122–141.
- 44. Katz N, Hale M, Morris D, Stauffer J. Morphine sulfate and naltrexone hydrochloride extended release capsules in patients with chronic osteoarthritis pain. Postgrad Med 2010; 122: 112–128.
- 45. Munera C, Drehobl M, Sessler NE, Landau C. A randomized, placebo‐controlled, double‐blinded, parallel‐group, 5‐week study of buprenorphine transdermal system in adults with osteoarthritis. J Opioid Manag 2010; 6: 193–202.
- 46. DeLemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended‐release once‐daily in the treatment of osteoarthritis of the knee and/or hip: a double‐blind, randomized, dose‐ranging trial. Am J Ther 2011; 18: 216–226.
- 47. Friedmann N, Klutzaritz V, Webster L. Efficacy and safety of an extended‐release oxycodone (Remoxy) formulation in patients with moderate to severe osteoarthritic pain. J Opioid Manag 2011; 7: 193–202.
- 48. Vojtaššák J, Vojtaššák J, Jacobs A, et al. A phase IIIb, multicentre, randomised, parallel‐group, placebo‐controlled, double‐blind study to investigate the efficacy and safety of OROS hydromorphone in subjects with moderate‐to‐severe chronic pain induced by osteoarthritis of the hip or the knee. Pain Res Treat 2011; 2011: 239501.
- 49. Rauck R, Rapoport R, Thipphawong J. Results of a double‐blind, placebo‐controlled, fixed‐dose assessment of once‐daily OROS hydromorphone ER in patients with moderate to severe pain associated with chronic osteoarthritis. Pain Pract 2013; 13: 18–29.
- 50. Spierings EL, Fidelholtz J, Wolfram G, et al. A phase III placebo‐ and oxycodone‐controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain 2013; 154: 1603–1612.
- 51. Mayorga AJ, Wang S, Kelly KM, Thipphawong J. Efficacy and safety of fulranumab as monotherapy in patients with moderate to severe, chronic knee pain of primary osteoarthritis: a randomised, placebo‐ and active‐controlled trial. Int J Clin Pract 2016; 70: 493–505.
- 52. Tominaga Y, Koga H, Uchida N, et al. Methodological issues in conducting pilot trials in chronic pain as randomized, double‐blind, placebo‐controlled studies. Drug Res (Stuttg) 2016; 66: 363–370.
- 53. Serrie A, Lange B, Steup A. Tapentadol prolonged‐release for moderate‐to‐severe chronic osteoarthritis knee pain: a double‐blind, randomized, placebo‐ and oxycodone controlled release‐controlled study. Curr Med Res Opin 2017; 33: 1423–1432.
- 54. Abdel Shaheed C, McLachlan AJ, Maher CG. Rethinking “long term” opioid therapy. BMJ 2019; 367: l6691.
- 55. Xie J, Turkiewicz A, Collins G, et al. OP0280 temporal trends of opioid use among incident osteoarthritis patients in Catalonia, 2007–2016: a population based cohort study [abstract]. Ann Rheum Dis 2020; 79 (Suppl 1): 174–175.
- 56. Mundipharma. Targin consumer medicines information leaflet. Updated Aug 2020. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP‐2010‐CMI‐04502‐3&d=202009181016933 (viewed Oct 2020).
- 57. Abdel Shaheed C, Ferreira GE, Dmitritchenko A, et al. The efficacy and safety of paracetamol for pain relief: an overview of systematic reviews. Med J Aust 2021; 214: 324–331. https://www.mja.com.au/journal/2021/214/7/efficacy‐and‐safety‐paracetamol‐pain‐relief‐overview‐systematic‐reviews
- 58. Machado GC, Maher CG, Ferreira PH, et al. Non‐steroidal anti‐inflammatory drugs for spinal pain: a systematic review and meta‐analysis. Ann Rheum Dis 2017; 76: 1269–1278.





Abstract
Objective: To evaluate the efficacy and safety of opioids for analgesic therapy for people with osteoarthritis.
Study design: Systematic review and meta‐analysis of randomised, placebo‐controlled trials of opioid therapies for treating the pain of osteoarthritis. The primary outcome was medium term pain relief (six weeks to less than 12 months). Quality of evidence was assessed with GRADE criteria.
Data sources: MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews and Central Register of Controlled Trials, CINAHL, PsycINFO, AMED, and the WHO International Clinical Trials Registry; trials published to 31 October 2020.
Data synthesis: We extracted pain, disability, health‐related quality of life, and adverse events data for 36 eligible trials (overall dose range: 10‒210 oral morphine milligram equivalents [MME] per day). Continuous pain and disability outcomes were converted to common 0–100‐point scales; changes of less than ten points were deemed to be very small effects. Differences in dichotomous outcomes were expressed as risk ratios. Data were pooled for meta‐analysis in random effects models. The evidence from 19 trials (8965 participants; dose range, 10–126 MME/day) for very small medium term pain relief (mean difference [MD], –4.59 points; 95% CI, –7.17 to –2.02 points) was low quality, as was that from 16 trials (6882 participants; dose range, 10–126 MME/day) for a very small effect on disability (MD, –4.15 points; 95% CI, –6.94 to –1.35 points). Opioid dose was not statistically significantly associated with either degree of pain relief or incidence of adverse events in a meta‐regression analysis. Evidence that opioid therapy increased the risk of adverse events (risk ratio, 1.43; 95% CI, 1.29‒1.59) was of very low quality.
Conclusions: Opioid medications may provide very small pain and disability benefits for people with osteoarthritis, but may also increase the risk of adverse events.
PROSPERO registration: CRD42019142813 (prospective).