The known: Clinical guidelines recommend that patients be referred to kidney specialists, to prepare for kidney failure or kidney replacement therapy, if their estimated glomerular filtration rate (eGFR) is persistently below 30 mL/min/1.73 m2.
The new: The risk of death for people over 65 with severe (stage 4) chronic kidney disease was greater than that of kidney failure, the risk of which declined with age. The risks of death and renal failure are each greater for older men than women with severe chronic kidney disease .
The implications: Clinical guidelines should recognise competing risks of death in older people with severe kidney disease, and consider holistic, supportive care, not just kidney replacement therapy.
Clinical guidelines recommend that a person with chronic kidney disease (CKD) be referred to a kidney specialist if their estimated glomerular filtration rate (eGFR) falls below 30 mL/min/1.73 m2 (stage 4 CKD).1 This pathway of care, managed by a multidisciplinary team, attempts to slow progression of disease, to avert and treat complications, and, crucially, to prepare patients for decisions about options for treating future kidney failure, including dialysis and transplantation.
The risks of cardiovascular disease events and death increase progressively with decline in eGFR: in one study, the adjusted hazard ratio for death at an eGFR of 15–29 mL/min/1.73 m2 was 3.2, and below 15 mL/min/1.73 m2 it was 5.9 (reference group: people with eGFR of 60 mL/min/1.73 m2 or more).2 High mortality rates for people with low eGFR mean that death is a competing risk for any other long term outcome.3,4 A Canadian group that recently examined the competing risks of death and kidney failure (eGFR < 10 mL/min/1.73 m2, initiation of dialysis, or kidney transplantation) in people with stage 4 CKD (eGFR, 15–30 mL/min/1.73 m2) found an age‐related increase in the likelihood of death and reduced likelihood of kidney failure.5
The aim of our study was to explore the competing risks of death from any cause and from kidney failure in a cohort of Australian adults with severe kidney disease, both to facilitate comparison with overseas findings and to inform local clinical practice.
Methods
The Tasmanian Chronic Kidney Disease study (CKD.TASlink) was a retrospective cohort study that analysed a dataset compiled by linking seven local health information datasets. The methodology has been reported in detail elsewhere.6 Briefly, pathology data for 490 012 people during the period 1 January 2004 – 31 December 2017 were obtained from four community and hospital‐based pathology providers (Hobart Pathology, Launceston Pathology, North West Pathology, Royal Hobart Hospital Pathology; two smaller pathology providers were not included). Individuals were selected if assessment of serum creatinine level had been requested by their doctors. Our study population included an estimated 86.8% of the resident adult population and about 97.5% of deaths in Tasmania during the final five years of the study, 2013–2017.6,7
Study entry: chronic kidney disease
All Tasmanian adults (aged 18 years or older) with stage 4 CKD were eligible for inclusion. Stage 4 CKD was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) definition: two recorded eGFR values of 15–29 mL/min/1.73 m2 more than 90 days apart, with eGFR based on serum creatinine (eGFRcreat) calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) creatinine equation and creatinine calibrated to isotope dilution–mass spectrometry.1,8,9
The criterion for stage 4 CKD was a mean eGFR of 15–29 mL/min/1.73 m2. We calculated the mean eGFR from at least two measurements more than 90 days apart, commencing with the date of the first value below 30 mL/min/1.73 m2. We defined the date of cohort entry (index date) as that of the final eGFR measurement included in the calculation of the mean value (index eGFR). When multiple eGFR measurements were available for a single day, we included the lowest value.
We excluded people who had undergone kidney replacement therapy before study entry, and those with one or more eGFR readings below 15 mL/min/1.73 m2 (ie, stage 5 CKD) before the episode that qualified them for study entry. We did not exclude people who may have had acute kidney injury, but we calculated a 3‐month rolling mean for inclusion and required a minimum of two years’ follow‐up, which would exclude most people with short term reductions in eGFR.
We used the most recently reported urinary albumin:creatinine ratio for assessing albuminuria (on or preceding the index date), categorised as normal (< 30 mg/g), microalbuminuria (30–300 mg/g), macroalbuminuria (> 300 mg/g), or unmeasured.1,10
Kidney failure
We defined the endpoint of kidney failure as eGFR below 10 mL/min/1.73 m2 (mean value over more than 90 days). We calculated the mean eGFR from at least two eGFR measurements over a period of more than 90 days from the date of the first eGFR value below 10 mL/min/1.73 m2; when multiple eGFR measurements were available for a single day, we included the lowest value. We defined the event date as that of the final eGFR measurement during this period. The endpoint of kidney failure was deemed not to have been reached if only one eGFR measurement below 10 mL/min/1.73 m2 was recorded or people did not have two values below 10 mL/min/1.73 m2 more than 90 days apart; such individuals were deemed event‐free, and remained “at risk”. People who died after single eGFR measurements below 10 mL/min/1.73 m2 were classified as not having reached the endpoint of kidney failure unless their death was attributable to kidney failure, as indicated by International Statistical Classification of Diseases, tenth revision, Australian modification (ICD‐10‐AM) cause of death codes included in the Elixhauser comorbidity group “renal failure”11 (Supporting Information, table 1). As we did not have information about people leaving Tasmania, we censored observations at 18 months after the last recorded eGFR measurement, to minimise selection bias.
Primary outcome and independent variables
The primary outcomes of our study were kidney failure or first use of kidney replacement therapy, as reported to the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA; https://www.anzdata.org.au/anzdata), and death, as recorded in the Tasmanian deaths registry. We report the risks of the primary outcome, expressed as a decimal number between 0 and 1, during the five years following diagnosis of stage 4 CKD.
Diabetes was identified in inpatient episode and cause of death records on the basis of ICD‐10‐AM diagnosis codes in relevant Elixhauser comorbidity groups (E10–E14; (Supporting Information, table 2), pathology data (glycosylated haemoglobin [HbA1c] > 48 mmol/mol or fasting glucose > 7.0 mmol/L), or recorded diabetes‐related cause of death. Cardiovascular disease (CVD) was identified on the basis of ICD‐10 diagnosis codes in relevant Elixhauser comorbidity groups (Supporting Information, table 3) and Australian Classification of Health Interventions CVD procedure codes (for people with admitted patient episodes; Supporting Information, table 4), or recorded CVD‐related cause of death. Only inpatient episodes, diagnoses, and procedures prior to or on the index date and during the subsequent five years were included. The lookback period for inclusion was to the first appearance of an individual in the dataset (maximum of eleven years prior to the index date). Age was defined as age at the diagnosis of stage 4 CKD.
Statistical analysis
We assessed associations of age group with the risks of kidney failure and death using the methodology described by Ravani and colleagues,5 to facilitate comparisons with overseas findings. We estimated crude cumulative incidence functions — the probability of an event attributable to a specific cause k at time t — for kidney failure and for death without kidney failure, with 95% confidence intervals (CIs), by age category.
We tested the consistency of our findings in sensitivity analyses stratified by index eGFR value at cohort entry, with different definitions of kidney failure (eGFR < 10 mL/min/1.73 m2 and initiation of kidney replacement therapy; or kidney replacement therapy alone), or restricted to people for whom complete albuminuria testing results were available.
Ethics approval
The CKD.TASlink protocol was reviewed and approved by the Tasmanian Human Research Ethics Committee (approved study H0016499).
Results
A total of 461 484 adults in Tasmania with serum creatinine measurements during 2004–2017 were included in the CKD.TASlink study population, of whom 6825 people met the inclusion criteria, including 3816 women (55.9%) (Supporting Information, figure 1). During the stage 4 CKD detection window (2004–2015), 701 people commenced renal replacement therapy (ANZDATA registry data), of whom 275 had not been identified as having stage 4 CKD (39%). Median eGFR at commencement of kidney replacement therapy was 7.0 mL/min/1.73 m2 (interquartile range, 5.2–9.4 mL/min/1.73 m2).
Fifty‐six per cent of people with stage 4 CKD under 65 years of age were men, whereas 58% of those aged 75 years or more were women. The urine albumin:creatinine ratio had been measured for 1942 people (28.5%), of whom 703 had normal values (36%), 684 microalbuminuria (35%), and 555 macroalbuminuria (29%). Of 6825 included people, 2417 had diabetes (35.4%) and 3918 CVD (57.4%); the proportions with diabetes (50.2%) and albuminuria (81% of those with measured values) were each highest for people under 65 years of age (Box 1).
The annual incidence of stage 4 CKD declined in each age group during 2004–2007 and has since been relatively stable; it increased markedly with age (Box 2). However, kidney replacement therapy was relatively infrequent among people aged 85 years or more (Supporting Information, figure 2, table 5).
Crude risks of kidney failure or death
For people under 65 years of age, the crude risk of death during the five years following diagnosis of stage 4 CKD was 0.18 (95% CI, 0.15–0.22) and that of kidney failure 0.39 (95% CI, 0.35–0.43). The risk of death increased with age — 65–74 years: 0.39 (95% CI, 0.36–0.42); 75–84 years: 0.56 (95% CI, 0.54–0.58); 85 years or more: 0.78 (95% CI, 0.77–0.80) — while that of kidney failure diminished — 65–74 years: 0.12 (95% CI, 0.10–0.14); 75–84 years: 0.05 (95% CI, 0.04–0.06); 85 years or more: 0.01 (95% CI, 0.01–0.02). For people over 75 years of age, the risk of kidney failure before dying was 0.05 (95% CI, 0.04–0.06) (Box 3).
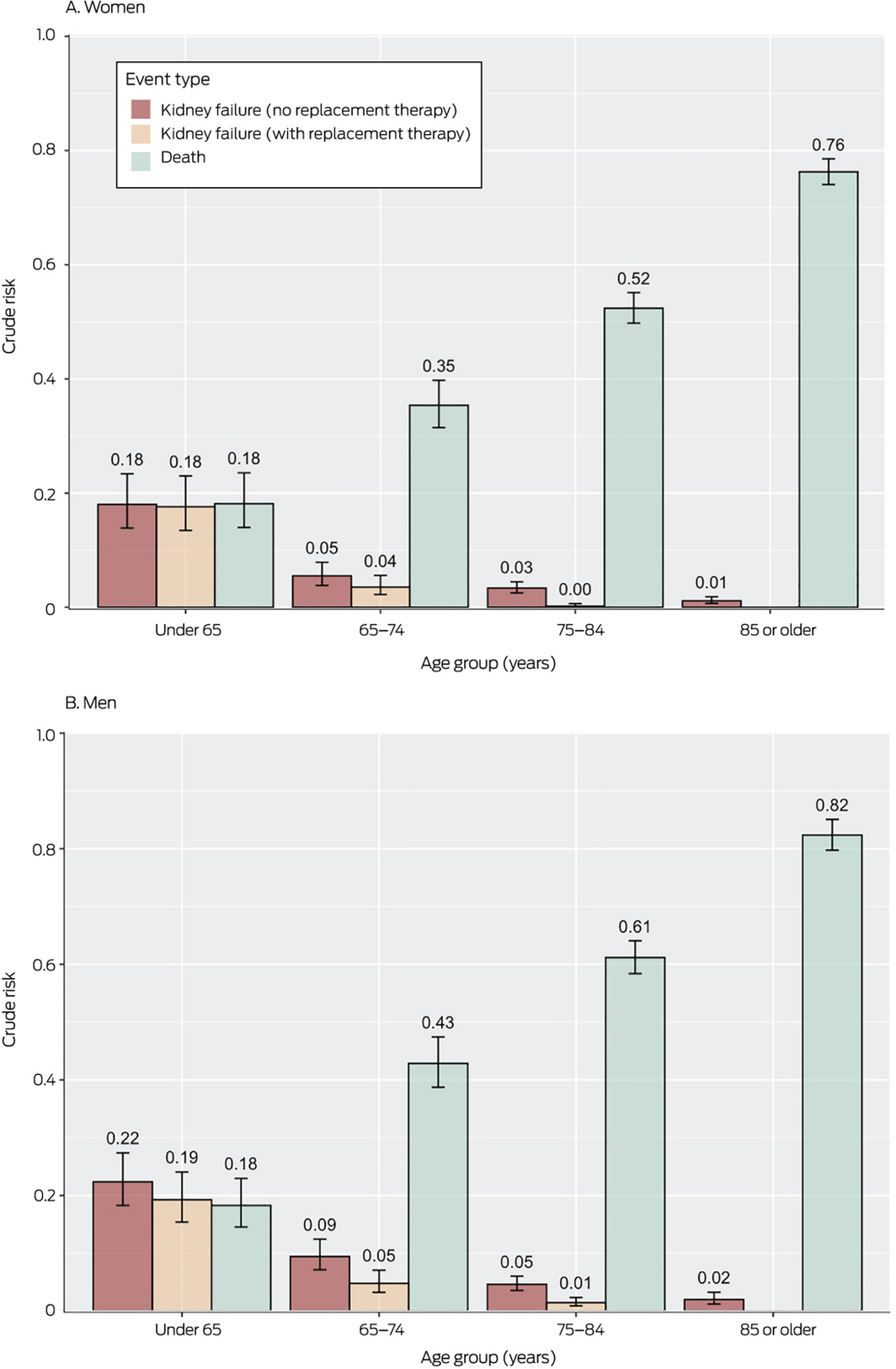
Crude risks of kidney failure or death, by sex
The 5‐year risk of kidney failure was lower for women than men in all age groups; the 5‐year risk of death was also lower for women, except for those under 65 years of age. The risks of kidney failure with kidney replacement therapy were similar for men and women in all age groups (Box 4).
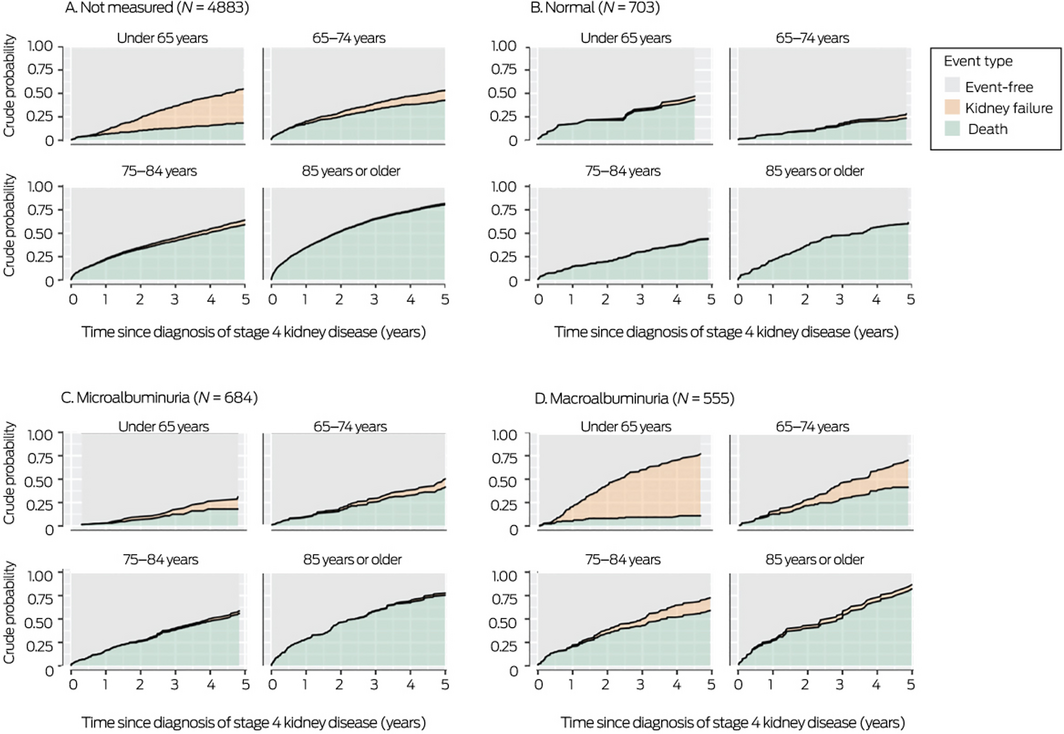
Crude risks of kidney failure or death, by albuminuria status
The 5‐year risk of kidney failure was very low for people with a urine albumin:creatinine ratio in the normal range and high for those with macroalbuminuria. The risk of kidney failure was also substantial among people whose urine albumin:creatinine ratio had not been assessed, especially those under 65 years of age (Box 5).
Risks of kidney failure or death, stratified by comorbid diabetes and cardiovascular disease
For all age groups, risk profiles stratified by comorbid diabetes and CVD were similar. Among people aged 75–84 years, the 5‐year risk of death was higher than that of kidney failure for those with diabetes but not CVD (0.41; 95% CI, 0.35–0.47 v 0.04; 95% CI, 0.03–0.07) or CVD but not diabetes (0.65; 95% CI, 0.62–0.69 v 0.05; 95% CI, 0.03–0.06). Among people aged 85 years or more, the risk of death was higher than that of kidney failure for those without diabetes or CVD (0.66; 95% CI, 0.62–0.70 v 0.02; 95% CI, 0.01–0.03), with diabetes only (0.69; 95% CI, 0.63–0.76 v 0.01; 95% CI, 0.00–0.04), or CVD only (0.86; 95% CI, 0.84–0.88 v 0.01; 95% CI, 0.0–0.02) (Box 6; Supporting Information, table 6).
Sensitivity analyses
The results of our sensitivity analyses, in which we varied the definition of kidney failure, were similar to those of our main analyses (Supporting Information, figures 3–5). We also examined risk of death, stratified by final eGFR value prior to death; kidney failure was reported as the cause of death for very few people, including for those with eGFR values below 10 mL/min/1.73 m2 (Box 7; Supporting Information, figures 6 and 7).
Discussion
For Australian adults, age is a critical factor for the probability of kidney failure within five years of diagnosis with stage 4 CKD. Three important conclusions can be drawn from our study: for people with stage 4 CKD, the 5‐year risk of kidney failure declines with age; the risk of death is greater than that of kidney failure for those over 65 years of age; and the risks of death and kidney failure for people over 65 are each greater for men than women.
The relative importance of some mortality risk factors diminishes with age, while that of others, such as frailty, increases.12,13 This was reflected in our study: people over 65 years of age with stage 4 CKD were more likely to die than to experience kidney failure, suggesting that more people die with CKD rather than directly because of CKD. Organ‐specific guidelines, however, often do not take age into account, including those for the care of people with CKD.14 As kidney replacement therapy pathways are well defined and supported, disease management typically focuses on dialysis or transplantation; the pathway to supportive (non‐dialysis) care is less well defined, and differences between renal units are greater.15 As we concluded in an earlier study, mortality risks should be considered when discussing future kidney failure treatment options for patients over 65 years of age.16
We found that men over the age of 65 years were more likely than women to progress to kidney failure and to die within five years of diagnosis with stage 4 CKD; younger men were also more likely to experience kidney failure, but the risk of death was similar for both sexes. This finding is consistent with general population mortality rate differences between men and women, as well as the observation that CKD progresses more slowly in women.17 Treatment outcomes should therefore be reported by sex, and sex‐specific interventions developed.
Proteinuria was an important predictor of outcomes in our study: the 5‐year risk of kidney failure was greatest for people under 75 years of age with stage 4 CKD who had macroalbuminuria, and was very low for people without albuminuria or with microalbuminuria. Further, a considerable proportion of people under 65 for whom the urine albumin:creatinine ratio had not been assessed during the same calendar year as their diagnosis experienced kidney failure. This highlights the need to optimise the assessment of urinary albumin for the early detection and management of CKD.
Our findings generally concur with those of a recent Canadian study,5 although the 5‐year risk of kidney failure was lower in Tasmania than in Canada for all age groups. Further, the population risk of kidney failure without kidney replacement therapy was greater than that of failure with replacement therapy in Tasmania. In Canada, kidney replacement therapy was much more frequent among people with kidney failure; the proportion who experienced kidney failure without replacement therapy was larger than that of those who had received it only for people aged 85 years or more.5 The reasons for these differences are unknown, but could be related to differences in health care systems or population characteristics. The relatively rural nature of Tasmania may have influenced treatment choices; although kidney replacement therapy is readily available, the need for travel and perceived access difficulties may dampen uptake.
Limitations
The usual limitations of studies based on administrative datasets applied to our investigation. We did not have reliable data about ethnic background, and the applicability of our findings to Aboriginal and Torres Strait Islander people is consequently unknown. As we did not have information about people leaving Tasmania, we estimated loss to follow‐up by censoring data at 18 months after the last recorded eGFR result. Comorbid conditions were identified on the basis of recorded ICD‐10‐AM codes or pathology data, not diagnoses by medical practitioners. We did not have data for the 275 of 701 people commencing kidney replacement therapy without documented stage 4 CKD.
Conclusion
In this cohort of people in Australia with incident stage 4 CKD, the risk of death was greater than that of kidney failure for those aged 65 years or more. The 5‐year risk of kidney failure declined with age, and for people over 65 the risks of kidney failure and death were each greater for men than women. Clinical guidelines should recognise the competing risks of death from any cause and of kidney failure, and provide recommendations about holistic, supportive care rather than focusing on kidney replacement therapy alone.
Box 1 – Baseline characteristics of the 6825 people with stage 4 chronic kidney disease included in our analysis
|
|
Age (years) |
||||||||||||||
|
Characteristic |
Under 65 |
65–74 |
75–84 |
85 or more |
All ages |
||||||||||
|
|
|||||||||||||||
|
Number of people |
622 |
1083 |
2720 |
2400 |
6825 |
||||||||||
|
Age (years) |
|
|
|
|
|
||||||||||
|
Mean (SD) |
54.1 (9.5) |
70.3 (2.8) |
80.0 (2.8) |
89.2 (3.4) |
79.3 (11.1) |
||||||||||
|
Median (IQR) |
57.0 (49.3–61.0) |
71.0 (68.0–73.0) |
80.0 (78.0–82.0) |
89.0 (86.0–91.0) |
81.0 (75.0–87.0) |
||||||||||
|
Sex |
|
|
|
|
|
||||||||||
|
Women |
276 (44.4%) |
546 (50.4%) |
1477 (54.3%) |
1517 (63.2%) |
3816 (55.9%) |
||||||||||
|
Men |
346 (55.6%) |
537 (49.6%) |
1243 (45.7%) |
883 (36.8%) |
3009 (44.1%) |
||||||||||
|
eGFR (mL/min/1.73 m2) |
|
|
|
|
|
||||||||||
|
Mean (SD) |
27.4 (2.8) |
27.8 (2.4) |
27.7 (2.5) |
27.5 (2.6) |
27.6 (2.5) |
||||||||||
|
Median (IQR) |
28.3 (26.4–29.4) |
28.6 (26.9–29.5) |
28.6 (27.0–29.4) |
28.3 (26.5–29.3) |
28.5 (26.7–29.4) |
||||||||||
|
Albuminuria* |
|
|
|
|
|
||||||||||
|
Normal |
56 (9.0%) |
150 (13.9%) |
327 (12.0%) |
170 (7.1%) |
703 (10.3%) |
||||||||||
|
Microalbuminuria |
65 (10%) |
155 (14.3%) |
299 (11.0%) |
165 (6.9%) |
684 (10.0%) |
||||||||||
|
Macroalbuminuria |
173 (27.8%) |
134 (12.4%) |
180 (6.6%) |
68 (2.8%) |
555 (8.1%) |
||||||||||
|
Unmeasured |
328 (52.7%) |
644 (59.5%) |
1914 (70.4%) |
1997 (83.2%) |
4883 (71.5%) |
||||||||||
|
Diabetes |
|
|
|
|
|
||||||||||
|
Yes |
312 (50.2%) |
538 (49.7%) |
972 (35.7%) |
595 (24.8%) |
2417 (35.4%) |
||||||||||
|
No |
310 (49.8%) |
545 (50.3%) |
1748 (64.3%) |
1805 (75.2%) |
4408 (64.6%) |
||||||||||
|
Cardiovascular disease |
|
|
|
|
|
||||||||||
|
Yes |
273 (43.9%) |
573 (52.9%) |
1583 (58.2%) |
1489 (62.0%) |
3918 (57.4%) |
||||||||||
|
No |
349 (56.1%) |
510 (47.1%) |
1137 (41.8%) |
911 (38.0%) |
2907 (42.6%) |
||||||||||
|
|
|||||||||||||||
|
eGFR = estimated glomerular filtration rate; IQR = interquartile range; SD = standard deviation. * Urine albumin:creatinine ratio: normal, < 30 mg/g; microalbuminuria, 30–300 mg/g; macroalbuminuria, > 300 mg/g. |
|||||||||||||||
Box 2 – Incidence of stage 4 chronic kidney disease, by age group and year*
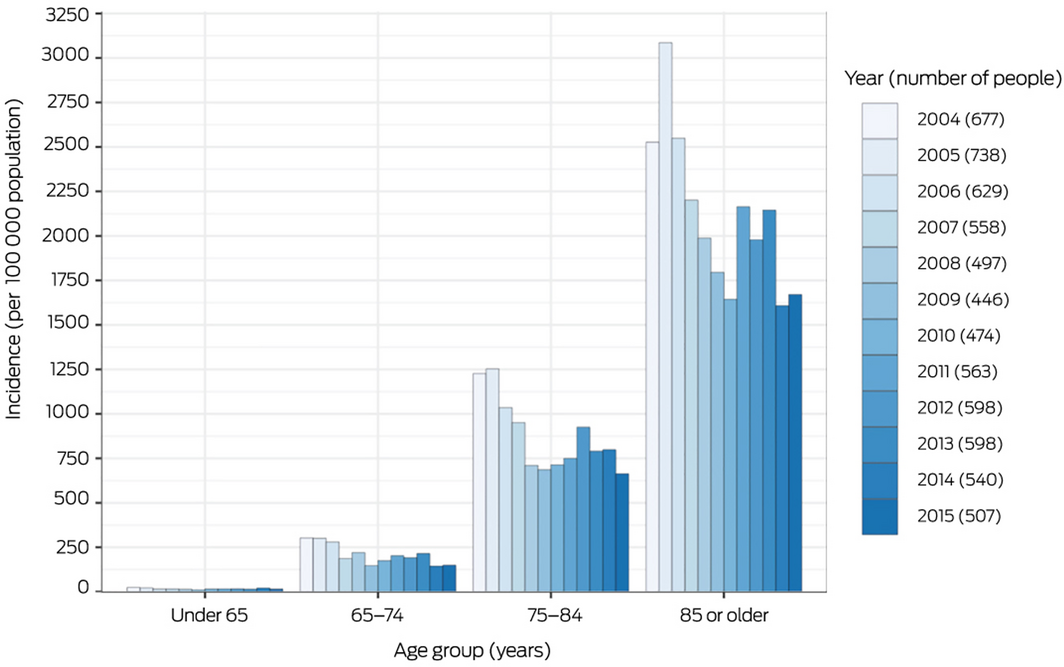
* The numerator is the number of people with a 90‐day moving mean eGFR of 15–29 mL/min/1.73 m2; the denominator is the estimated Tasmanian resident population for each age group.7 Incidence for 2016 and 2017 are not included as we required a minimum of two years’ follow‐up from diagnosis of stage 4 chronic kidney disease.
Box 3 – Crude risks of kidney failure or death during the five years following diagnosis of stage 4 kidney disease, by age group*
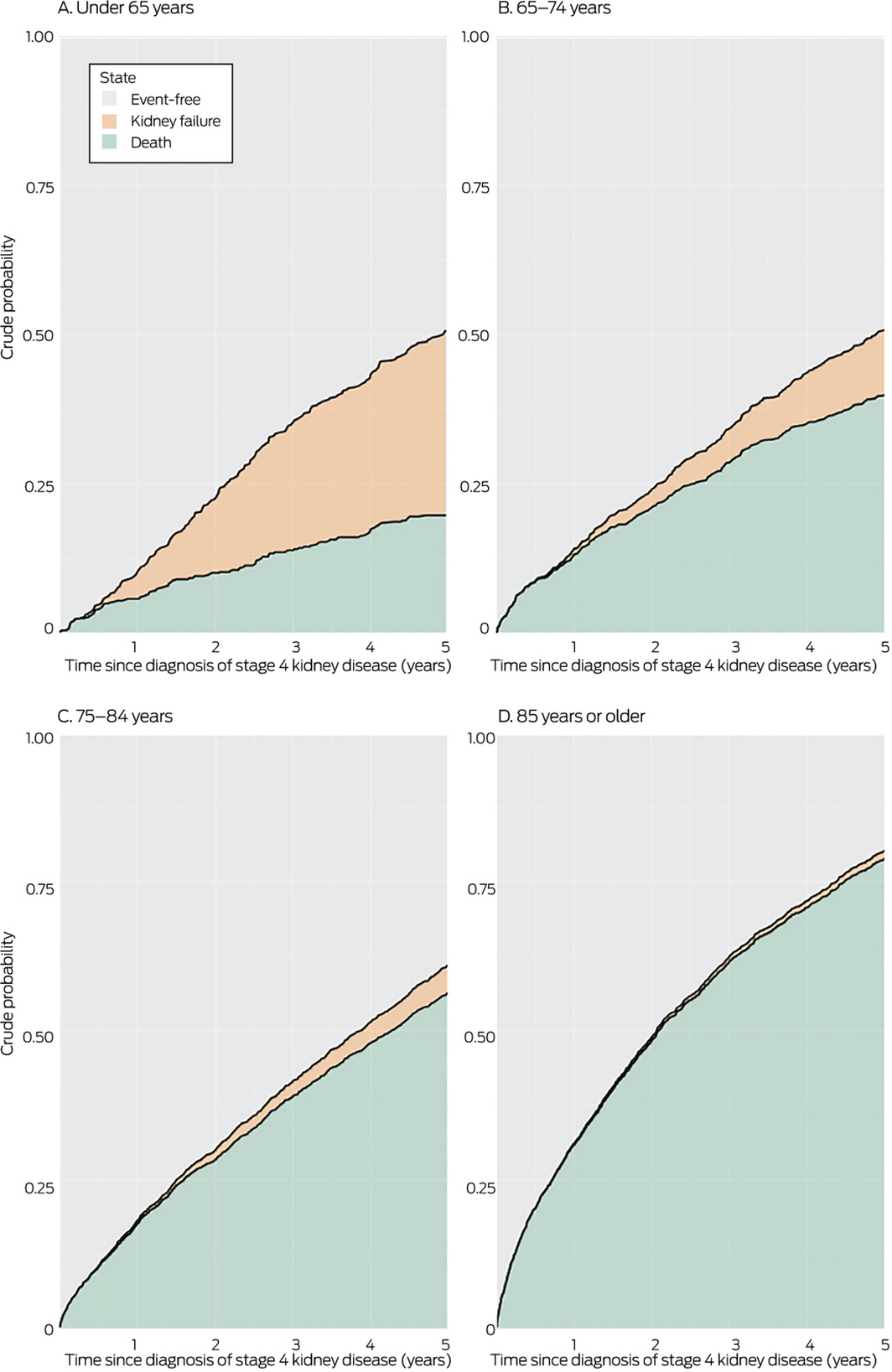
* Stage 4 chronic kidney disease defined as eGFR of 15–29 mL/min/1.73 m2; kidney failure defined as eGFR < 10 mL/min/1.73 m2 or commencement of kidney replacement therapy.
Box 4 – Crude risks of death or kidney failure (with or without kidney replacement therapy) within five years of diagnosis of stage 4 kidney disease, by sex and age group
Box 5 – Crude risks of death or kidney failure within five years of diagnosis of stage 4 kidney disease, by albuminuria status and age group
Box 6 – Probabilities and 5‐year risks of death and kidney failure within five years of diagnosis of stage 4 kidney disease, by age group and comorbid diabetes mellitus and cardiovascular disease (CVD)*
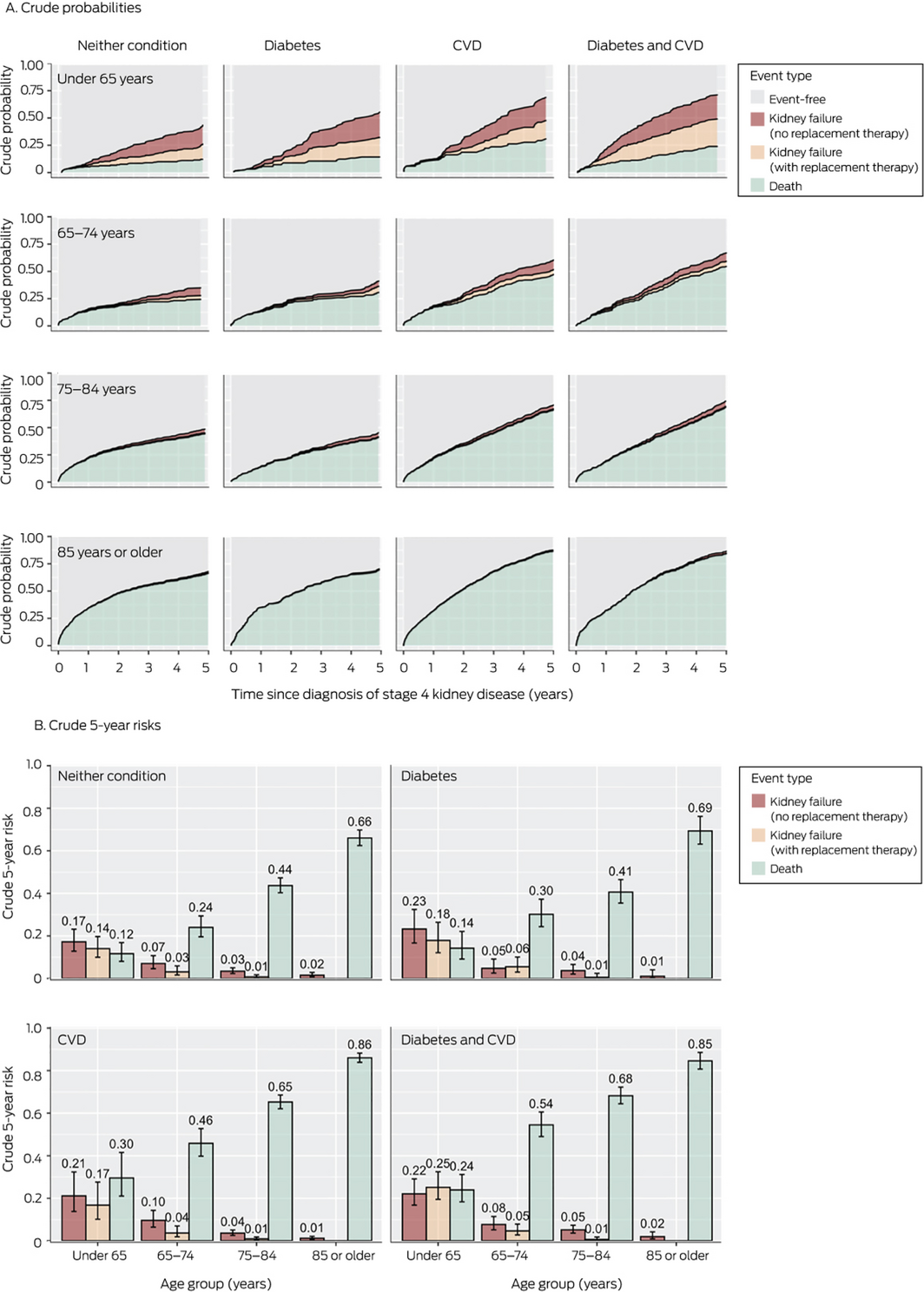
*For numbers of people in each category, see Supporting Information, table 6.
Box 7 – Outcomes during study period, with death stratified by final eGFR result prior to death
|
|
Age group (years) |
||||||||||||||
|
Outcome |
Under 65 |
65–74 |
75–84 |
85 or more |
|||||||||||
|
|
|||||||||||||||
|
Event‐free |
201 |
370 |
668 |
318 |
|||||||||||
|
Kidney failure without kidney replacement therapy |
157 |
113 |
151 |
41 |
|||||||||||
|
Kidney failure with kidney replacement therapy |
131 |
59 |
24 |
0 |
|||||||||||
|
Death after one eGFR < 10 mL/min/1.73 m2 |
19 |
39 |
131 |
75 |
|||||||||||
|
Kidney failure as cause of death within 90 days of first eGFR < 10 mL/min/1.73 m2 |
2 |
6 |
21 |
9 |
|||||||||||
|
Death with eGFR ≥ 10 mL/min/1.73 m2 |
116 |
508 |
1767 |
1975 |
|||||||||||
|
|
|||||||||||||||
|
eGFR = estimated glomerular filtration rate. |
|||||||||||||||
Received 8 January 2021, accepted 21 July 2021
- Matthew D Jose1,2
- Rajesh Raj3,4
- Kim Jose5
- Alex Kitsos1
- Tim Saunder1
- Charlotte McKercher5
- Jan Radford3
- 1 University of Tasmania, Hobart, TAS
- 2 Royal Hobart Hospital, Hobart, TAS
- 3 Launceston General Hospital, Launceston, TAS
- 4 University of Tasmania, Launceston, TAS
- 5 Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS
We thank Diagnostic Services and Pathology South for providing pathology data; the Tasmanian Department of Health for Tasmanian Public Hospital Admitted Patient and Emergency Department Presentations data; the Registry of Births, Deaths and Marriages (Tasmania), the Australian Coordinating Registry, and the National Coronial Information System for cause of death unit record file data; the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) for dialysis and transplantation data; and the Tasmanian Data Linkage Unit for undertaking linkage of these datasets. Some of the data reported were supplied by ANZDATA; the interpretation and reporting of these data are the responsibility of the authors and should not be regarded as official policy or an interpretation by ANZDATA.
No relevant disclosures.
- 1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl 2013; 3: 1–150.
- 2. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305.
- 3. Australian Institute of Health and Welfare. Deaths in Australia (Cat. no. PHE 229). Updated 7 Aug 2020. https://www.aihw.gov.au/reports/life‐expectancy‐death/deaths‐in‐australia (viewed Sept 2020).
- 4. Austin PC, Fine JP. Accounting for competing risks in randomized controlled trials: a review and recommendations for improvement. Stat Med 2017; 36: 1203–1209.
- 5. Ravani P, Quinn R, Fiocco M, et al. Association of age with risk of kidney failure in adults with stage IV chronic kidney disease in Canada. JAMA Netw Open 2020; 3: e2017150.
- 6. Saunder T, Kitsos A, Radford J, et al. Chronic kidney disease in Tasmania: protocol for a data linkage study. JMIR Res Protoc 2020; 9: e20160.
- 7. Australian Bureau of Statistics. ERP by SA2 (ASGS 2016), age and sex, 2001 onwards [dataset: reference period: 30 June 2001 – 30 June 2019. https://stat.data.abs.gov.au/Index.aspx?DataSetCode=ABS_ERP_ASGS2016 (viewed June 2021).
- 8. Levey AS, Stevens LA, Schmid CH, et al; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612.
- 9. Jose MD, Otahal P, Kirkland G, et al. Retrospective standardisation of serum creatinine identified changes in eGFR over time [abstract: Australian and New Zealand Society of Nephrology, 44th annual scientific meeting, Newcastle, Australia, 6–10 Sept 2008]. Nephrology 2008; 13 (Suppl 3): A101.
- 10. Royal Australasian College of Pathologists. Albumin urine (RCPA manual). https://www.rcpa.edu.au/Manuals/RCPA‐Manual/Pathology‐Tests/A/Albumin‐urine (viewed Oct 2021).
- 11. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998; 36: 8–27.
- 12. Goldman N, Glei DA, Weinstein M. The best predictors of survival: do they vary by age, sex, and race? Popul Dev Rev 2017; 43: 541–560.
- 13. House JS, Lepkowski JM, Kinney AM, et al. The social stratification of aging and health. J Health Soc Behav 1994; 35: 213–234.
- 14. O’Hare AM. How useful is an age‐neutral model of chronic kidney disease? JAMA Netw Open 2020; 3: e2017592.
- 15. Murtagh FE, Burns A, Moranne O, et al. Supportive care: comprehensive conservative care in end‐stage kidney disease. Clin J Am Soc Nephrol 2016; 11: 1909–1914.
- 16. Raj R, Brown B, Ahuja K, et al. Enabling good outcomes in older adults on dialysis: a qualitative study. BMC Nephrol 2020; 21: 28.
- 17. Piccoli GB, Alrukhaimi M, Liu ZH, et al; World Kidney Day Steering Committee. Women and kidney disease: reflections on World Kidney Day 2018. Kidney Int 2018; 93: 278–283.





Abstract
Objectives: To examine the competing risks of death (any cause) and of kidney failure in a cohort of Australian adults with severe chronic kidney disease.
Design: Population‐based cohort study; analysis of linked data from the Tasmanian Chronic Kidney Disease study (CKD.TASlink), 1 January 2004 – 31 December 2017.
Participants: All adults in Tasmania with incident stage 4 chronic kidney disease (estimated glomerular filtration rate [eGFR], 15‒29 mL/min/1.73 m2).
Main outcome measures: Death or kidney failure (defined as eGFR below 10 mL/min/1.73 m2 or initiation of dialysis or kidney transplantation) within five years of diagnosis of stage 4 chronic kidney disease.
Results: We included data for 6825 adults with incident stage 4 chronic kidney disease (mean age, 79.3 years; SD, 11.1 years), including 3816 women (55.9%). The risk of death increased with age — under 65 years: 0.18 (95% CI, 0.15–0.22); 65‒74 years: 0.39 (95% CI, 0.36‒0.42); 75‒84 years, 0.56 (95% CI, 0.54‒0.58); 85 years or older: 0.78 (95% CI, 0.77‒0.80) — while that of kidney failure declined — under 65 years: 0.39 (95% CI, 0.35–0.43); 65‒74 years: 0.12 (95% CI, 0.10‒0.14); 75‒84 years: 0.05 (95% CI, 0.04‒0.06); 85 years or older: 0.01 (95% CI, 0.01‒0.02). The risk of kidney failure was greater for people with macroalbuminuria and those whose albumin status had not recently been assessed. The risks of kidney failure and death were greater for men than women in all age groups (except similar risks of death for men and women under 65 years of age).
Conclusions: For older Australians with incident stage 4 chronic kidney disease, the risk of death is higher than that of kidney failure, and the latter risk declines with age. Clinical guidelines should recognise these competing risks and include recommendations about holistic supportive care, not just on preparation for dialysis or transplantation.