The known: Drug‐related anaphylaxis is a frequent cause of presentations to hospital emergency departments. Penicillin‐class antimicrobials are often implicated in allergic reactions.
The new: Newly mandatory reporting data provide a valuable snapshot of anaphylaxis in Victoria. Medications were implicated in 12% of emergency department presentations with anaphylaxis, 48% of which were antimicrobial‐related. Penicillins (56%) and cephalosporins (31%) were the most frequently implicated antimicrobial medications.
The implications: We have provided a unique snapshot of drug‐ and antimicrobial‐related anaphylaxis in Australia, for which representative data are limited. Understanding drug‐related anaphylaxis could improve prescribing practice in Australia.
Anaphylaxis is an acute, potentially life‐threatening condition caused by IgE‐mediated mast cell degranulation. The estimated lifetime prevalence of all‐cause anaphylaxis in the United States is about 2%,1,2 with an estimated incidence of about 30 episodes per 100 000 person‐years.3 The prevalence of anaphylaxis appears to be increasing,2,4 particularly among children.5
Estimating rates of drug‐related anaphylaxis is difficult, partly because of classification problems.6 Food allergy is the most frequent trigger of anaphylaxis during childhood, while drug‐related anaphylaxis increases with age, and is responsible for about 40% of all cases in the United States and the United Kingdom.2,7
From 1 November 2018, the Victorian Department of Health and Human Services listed anaphylaxis as a notifiable condition.8 The incidence, causes, and characteristics of anaphylaxis in a large Australian state could now be analysed for the first time. The aim of our analysis of data from the first two years of mandatory reporting was to determine the incidence of anaphylaxis reported from emergency departments in Victoria, particularly drug‐related anaphylaxis. We also examined clinical outcomes following anaphylaxis, including differences in presentation and outcome by drug class and antimicrobial agent sub‐class.
Methods
We reviewed all cases of anaphylaxis reported to the Victorian Department of Health (previously: Victorian Department of Health and Human Services) during 1 November 2018 – 31 December 2020. The Department collects demographic and outcomes data in a structured case report form for all cases of anaphylaxis in people presenting to Victorian public or private hospital emergency departments.8 Other cases of anaphylaxis, including episodes in hospitalised patients, are not notifiable.
The Department provided summary epidemiological data (counts, median age, outcomes) for all reported cases of anaphylaxis during 1 November 2018 – 31 December 2020. De‐identified, case‐level data for all cases of drug‐related anaphylaxis were also requested and provided, including patient age, sex, requirement for inpatient or intensive care unit admission, whether the case was one of first‐time anaphylaxis, death from anaphylaxis, and drug name and type. We analysed drug‐related anaphylaxis by class (eg, antimicrobial) and sub‐class (eg, cephalosporins).
Data quality was checked by epidemiologists at the Department of Health, and assessed for labelling consistency by author GPD. Data are routinely cleaned on receipt of notifications in order to correct misclassified causes, other obvious errors, and duplicate notifications. Further, Department of Health epidemiologists periodically audit the collated data for accuracy.
Definitions
There is no universally accepted definition of anaphylaxis. For reporting purposes, the Victorian Department of Health has based its definition on the Safer Care Victoria standard as a “severe, potentially life‐threatening systemic hypersensitivity reaction characterised by rapid onset of life‐threatening airway, breathing, or circulatory problems, and (usually, but not always) skin and mucosal changes … Vomiting and abdominal pain are symptoms of anaphylaxis to insect venom and systemically administered allergens.”9 Inpatient admission was defined as hospitalisation beyond emergency department care before discharge home.
Statistical analysis
Statistical analyses were performed in Stata MP 16.1. Continuous variable data were summarised as medians with interquartile ranges (IQRs), categorical data as counts and proportions. The statistical significance of differences in proportions was assessed in χ2 tests.
Ethics approval
All data were obtained and reported under the legislative authority of the Public Health and Wellbeing Act 2008 (Victoria). Formal ethics approval was not required as the analysis involved de‐identified data and constituted a departmental routine quality assurance activity for the notifiable conditions dataset.
Results
A total of 4273 anaphylaxis episodes were reported during the study period (females: 2292, 54%), including 4048 presentations to public hospital emergency departments (94.7%), 221 to private hospital emergency departments (5.2%), and four to emergency departments of unrecorded type (0.1%). For 1905 patients (45%) it was the first anaphylaxis episode; 1538 patients (36%) required hospitalisation, 111 intensive care (2.6%, or 7% of people admitted to hospital). The most frequently reported causes of anaphylaxis were foods (2659 cases, 62%) and “unknown” (595 cases, 14%). Drugs were the reported cause in 533 cases (12%), insect venoms in 342 (8%), and other causes in 144 (4%) (Box 1). No deaths were recorded.
The overall rate of anaphylaxis during 1 November 2018 – 31 December 2020 was 31.9 episodes per 100 000 person‐years. Rates of food‐related anaphylaxis were highest among people aged 0–39 years (median age, 17 years; IQR, 6–29 years), and those of drug‐related anaphylaxis among people aged 40–79 years (median age, 45 years; IQR, 30–60 years) (Box 2).
Drug‐related anaphylaxis
A total of 533 cases of drug‐related anaphylaxis were reported (females: 345, 65%). For 363 people (71%) it was the first episode of anaphylaxis of any cause; 233 people (47%) were admitted to hospital, including 30 who required intensive care (6%, or 13% of people admitted to hospital) (Box 3).
The drug classes most frequently implicated were antimicrobial medications (258 cases, 48%), non‐steroidal anti‐inflammatory drugs (NSAIDS) (85, 16%), herbals and supplements (32, 6%), and intravenous imaging contrast medium (32, 6%). Of the antimicrobial‐related reactions, the sub‐classes most frequently implicated were penicillins (143, 56%), cephalosporins (80, 31%), trimethoprim with or without cotrimoxazole (ten, 4%), metronidazole (seven, 3%), fluoroquinolones (six, 2%), and macrolides (four, 2%) (Box 4).
The proportions of people with drug‐related anaphylaxis admitted to hospital were similar for antimicrobial medications (131, 51%) and other medications (118, 43%; P = 0.20), as were the proportions admitted to intensive care (32, 12% v 39, 14%; P = 0.45). Larger proportions of reactions to penicillins were first anaphylaxis episodes (131, 92%) than of reactions to cephalosporins (62, 78%) or other antimicrobials (28, 79%).
Discussion
The mandatory reporting of emergency department presentations with anaphylaxis in Victoria has provided a unique opportunity for exploring a prospective dataset of information on causes and hospital outcomes. The rate of anaphylaxis we found was similar to that in the United States,3 but the rate of drug‐related anaphylaxis was lower than reported overseas.2,7 This may be partly explained by selection bias. Firstly, as anaphylaxis is only notifiable in Victoria when the person affected presents to an emergency department, our analysis does not include episodes affecting people already in hospital, where food‐ and insect‐related anaphylaxis are far less frequent. Secondly, overseas studies have found that anaphylaxis is underreported in emergency departments;10 this is different to the general underreporting of notifiable conditions when people present with less severe manifestations of disease,11 which is less likely for a condition such as anaphylaxis.
Antimicrobial medications and NSAIDs were the drug classes most frequently implicated in anaphylaxis, consistent with other reports.12 It is concerning that 6% of drug‐related cases were linked with herbal medications or supplements, for which drug regulation is often less rigorous than for registered pharmaceuticals.13,14
Our findings regarding antimicrobial‐related anaphylaxis are largely consistent with those of a retrospective multicentre cohort study in tertiary Australian hospitals during 2010–2015.15 Both studies found that mortality attributed to antimicrobial‐related anaphylaxis was very low (no deaths in our study, three deaths [1%] in the earlier study15), although 13% (our study) or 14%15 of patients required intensive care; the proportions of cases linked with cephalosporins were similar in our study (31%) and the 2010–2015 audit (35%).15 The larger proportion of penicillin‐related reactions that were instances of first‐time anaphylaxis than for other antimicrobial types suggests that people who had previously experienced penicillin allergy or anaphylaxis may subsequently be prescribed alternative agents.
Limitations
We analysed epidemiological surveillance and monitoring data, which include only limited demographic, clinical, and outcomes information. Nevertheless, our dataset included all cases of anaphylaxis in people who presented to Victorian emergency departments during the study period. As anaphylaxis is a serious condition that generally requires emergency department assessment and management, this probably includes almost all out‐of‐hospital cases of anaphylaxis. As a passive surveillance system, the Victorian anaphylaxis notification system is intrinsically prone to errors and omissions in data reporting. Further, the system does not require follow‐up of people with anaphylaxis (except food‐related anaphylaxis). Finally, as a retrospective, observational analysis, causal relationships cannot be inferred. However, as notification is mandatory, the descriptive sample in our study is representative of people who present with anaphylaxis to emergency departments in Victoria.
Conclusion
We reviewed prospectively collected data on anaphylaxis in non‐hospitalised people in Victoria. Our study provides a unique analysis of drug‐ and antimicrobial‐related anaphylaxis, for which prospective data are scarce; many studies have been based upon retrospective analyses of hospital stays. Understanding the reasons for and the outcomes of drug‐related anaphylaxis in Australia could improve decisions about prescribing and treatment.
Box 1 – Characteristics of all anaphylaxis cases notified from emergency departments, Victoria, 1 November 2018 – 31 December 2020
|
Characteristic |
Number |
||||||||||||||
|
|
|||||||||||||||
|
Total number of cases |
4273 |
||||||||||||||
|
Sex |
|
||||||||||||||
|
Female |
2292 (54%) |
||||||||||||||
|
Male |
1959 (46%) |
||||||||||||||
|
Not stated |
16 (< 1%) |
||||||||||||||
|
Other |
6 (< 1%) |
||||||||||||||
|
Suspected cause |
|
||||||||||||||
|
Blood‐derived product |
3 (< 1%) |
||||||||||||||
|
Food |
2659 (62%) |
||||||||||||||
|
Drug |
533 (12%) |
||||||||||||||
|
Insect venom |
342 (8%) |
||||||||||||||
|
Vaccine |
10 (< 1%) |
||||||||||||||
|
Other cause |
131 (3%) |
||||||||||||||
|
Unknown |
595 (14%) |
||||||||||||||
|
First‐time anaphylaxis |
|
||||||||||||||
|
Yes |
1905 (45%) |
||||||||||||||
|
No |
1747 (41%) |
||||||||||||||
|
Unknown |
621 (15%) |
||||||||||||||
|
Hospital admission |
|
||||||||||||||
|
Admitted |
1538 (36%) |
||||||||||||||
|
Not admitted |
1909 (45%) |
||||||||||||||
|
Unknown |
826 (19%) |
||||||||||||||
|
Intensive care unit admission* |
|
||||||||||||||
|
Admitted |
111 [7%] |
||||||||||||||
|
Not admitted |
1339 [87%] |
||||||||||||||
|
Unknown |
88 [6%] |
||||||||||||||
|
|
|||||||||||||||
|
* Proportions based on people admitted to hospital only. |
|||||||||||||||
Box 2 – Rates of anaphylaxis notified from emergency departments, Victoria, 1 November 2018 – 31 December 2020, by age group*
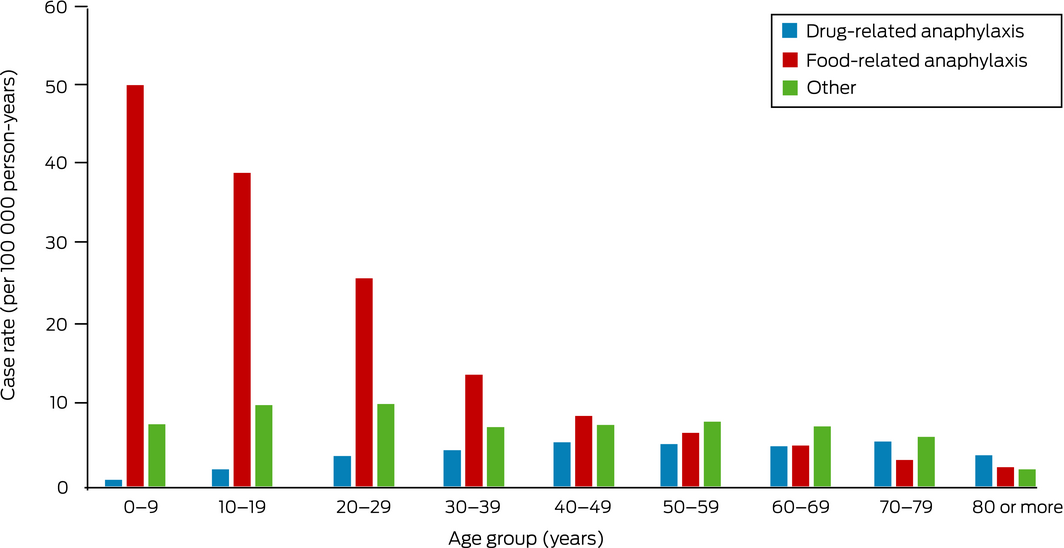
* The data for this graph are included in the Supporting Information file.
Box 3 – Characteristics of cases of drug‐related notified from emergency departments, Victoria, 1 November 2018 – 31 December 2020
|
Characteristic |
Number |
||||||||||||||
|
|
|||||||||||||||
|
Total number of cases |
533 |
||||||||||||||
|
Admitted to hospital |
|
||||||||||||||
|
Yes |
233 (47%) |
||||||||||||||
|
No |
204 (41%) |
||||||||||||||
|
Not stated |
56 (11%) |
||||||||||||||
|
First‐time anaphylaxis |
|
||||||||||||||
|
Yes |
363 (71%) |
||||||||||||||
|
No |
82 (16%) |
||||||||||||||
|
Not stated |
67 (13%) |
||||||||||||||
|
Admitted to intensive care* |
|
||||||||||||||
|
Yes |
30 [13%] |
||||||||||||||
|
No |
195 [85%] |
||||||||||||||
|
Not stated |
4 [2%] |
||||||||||||||
|
|
|||||||||||||||
|
* Proportions based on people admitted to hospital only. |
|||||||||||||||
Box 4 – Drugs implicated in cases of drug‐related anaphylaxis notified from emergency departments, Victoria, 1 November 2018 – 31 December 2020
|
Drug type |
Number |
||||||||||||||
|
|
|||||||||||||||
|
All drug‐related anaphylaxis |
533 |
||||||||||||||
|
Antimicrobial medications* |
258 (48.4%) |
||||||||||||||
|
Penicillins |
143 (26.8%) |
||||||||||||||
|
Amoxicillin |
84 (16%) |
||||||||||||||
|
Amoxicillin/clavulanic acid |
33 (6.2%) |
||||||||||||||
|
Phenoxymethylpenicillin |
9 (2%) |
||||||||||||||
|
Other/unspecified |
17 (3.2%) |
||||||||||||||
|
Cephalosporins |
80 (15%) |
||||||||||||||
|
Cefalexin |
54 (10%) |
||||||||||||||
|
Cefazolin |
9 (2%) |
||||||||||||||
|
Ceftriaxone |
9 (2%) |
||||||||||||||
|
Other/unspecified |
8 (2%) |
||||||||||||||
|
Other antimicrobials |
40 (7.5%) |
||||||||||||||
|
Trimethoprim with or without sulfamethoxazole |
10 (2%) |
||||||||||||||
|
Metronidazole |
7 (1%) |
||||||||||||||
|
Fluoroquinolone |
6 (1%) |
||||||||||||||
|
Ciprofloxacin |
5 (1%) |
||||||||||||||
|
Macrolides |
4 (1%) |
||||||||||||||
|
Doxycycline |
2 (< 1%) |
||||||||||||||
|
Glycopeptides |
2 (< 1%) |
||||||||||||||
|
Clindamycin |
1 (< 1%) |
||||||||||||||
|
Other |
3 (1%) |
||||||||||||||
|
Other medications |
275 (51.6%) |
||||||||||||||
|
Non‐steroidal anti‐inflammatory drugs |
85 (16%) |
||||||||||||||
|
Intravenous contrast medium |
32 (6.0%) |
||||||||||||||
|
Herbal medication/supplement |
32 (6.0%) |
||||||||||||||
|
Opioid |
18 (3.4%) |
||||||||||||||
|
Intravenous iron |
13 (2.4%) |
||||||||||||||
|
Anaesthetic |
7 (1%) |
||||||||||||||
|
Antitussive |
7 (1%) |
||||||||||||||
|
Proton pump inhibitor |
5 (1%) |
||||||||||||||
|
Angiotensin‐converting enzyme inhibitor |
4 (1%) |
||||||||||||||
|
Chemotherapeutic |
4 (1%) |
||||||||||||||
|
Antihistamine |
3 (1%) |
||||||||||||||
|
Triptans |
3 (1%) |
||||||||||||||
|
Antidepressant |
2 (< 1%) |
||||||||||||||
|
Antipsychotic |
2 (< 1%) |
||||||||||||||
|
Local anaesthetic |
2 (< 1%) |
||||||||||||||
|
Anti‐emetic |
1 (< 1%) |
||||||||||||||
|
Corticosteroid |
1 (< 1%) |
||||||||||||||
|
Other |
51 (9.6%) |
||||||||||||||
|
Unknown |
3 (1%) |
||||||||||||||
|
|
|||||||||||||||
|
* Two antimicrobial medications were implicated in each of five cases of anaphylaxis: one instance each of cefalexin and metronidazole, ceftriaxone and doxycycline, ceftriaxone and azithromycin, ciprofloxacin and metronidazole, and clarithromycin and amoxicillin. |
|||||||||||||||
Received 29 June 2021, accepted 22 December 2021
- George P Drewett1,2
- Jess Encena2
- Joy Gregory2
- Lucinda Franklin2
- Jason A Trubiano1,3
- 1 Austin Health, Melbourne, VIC
- 2 Victorian Department of Health, Melbourne, VIC
- 3 Peter MacCallum Cancer Institute, Melbourne, VIC
We acknowledge the contribution of Paul Goldsmith of the Food Safety Unit, Victorian Department of Health, for his work overseeing the anaphylaxis notifications and dataset.
No relevant disclosures.
- 1. Lieberman P, Camargo CA, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006; 97: 596–602.
- 2. Wood RA, Camargo CA, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol 2014; 133: 461–467.
- 3. Yang MS, Kim JY, Kim BK, et al. True rise in anaphylaxis incidence: epidemiologic study based on a national health insurance database. Medicine (Baltimore) 2017; 96: e5750.
- 4. Jeong K, Lee JD, Kang DR, Lee S. A population‐based epidemiological study of anaphylaxis using national big data in Korea: trends in age‐specific prevalence and epinephrine use in 2010–2014. Allergy Asthma Clin Immunol 2018; 14: 31.
- 5. Speakman S, Kool B, Sinclair J, Fitzharris P. Paediatric food‐induced anaphylaxis hospital presentations in New Zealand. J Paediatr Child Health 2018; 54: 254–259.
- 6. Tuttle KL, Wickner P. Capturing anaphylaxis through medical records: are ICD and CPT codes sufficient? Ann Allergy Asthma Immunol 2020; 124: 150–155.
- 7. González‐Pérez A, Aponte Z, Vidaurre CF, Rodríguez LA. Anaphylaxis epidemiology in patients with and patients without asthma: a United Kingdom database review. J Allergy Clin Immunol 2010; 125: 1098–1104.e1.
- 8. Victorian Department of Health. Anaphylaxis notifications. Updated 22 Oct 2021. https://www2.health.vic.gov.au/public‐health/anaphylaxis‐notifications (viewed Feb 2022).
- 9. Victorian Department of Health and Human Services. Anaphylaxis notifications under the Public Health and Wellbeing Act 2008: a guide for Victorian hospitals. 2019. https://www2.health.vic.gov.au/Api/downloadmedia/%7B51FA10E9‐E075‐4EA7‐88C1‐2B6E2537B262%7D (viewed Feb 2022).
- 10. Gaeta TJ, Clark S, Pelletier AJ, Camargo CA. National study of US emergency department visits for acute allergic reactions, 1993 to 2004. Ann Allergy Asthma Immunol 2007; 98: 360–365.
- 11. Hall G, Yohannes K, Raupach J, et al. Estimating community incidence of Salmonella, Campylobacter, and Shiga toxin‐producing Escherichia coli infections, Australia. Emerg Infect Dis 2008; 14: 1601–1609.
- 12. Regateiro FS, Marques ML, Gomes ER. Drug‐induced anaphylaxis: an update on epidemiology and risk factors. Int Arch Allergy Immunol 2020; 181: 481–487.
- 13. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health 2015; 105: 478–485.
- 14. Thakkar S, Anklam E, Xu A, et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul Toxicol Pharmacol 2020; 114: 104647.
- 15. Hall V, Wong M, Munsif M, et al. Antimicrobial anaphylaxis: the changing face of severe antimicrobial allergy. J Antimicrob Chemother 2020; 75: 229–235.





Abstract
Objective: To investigate the causes, characteristics, and outcomes of anaphylaxis, particularly drug‐related anaphylaxis, in Victoria during the first two years of mandatory notification.
Design: Review of all anaphylaxis cases reported by emergency departments to the Victorian Department of Health and Human Services.
Setting, participants: People presenting to all public and private hospital emergency departments in Victoria, 1 November 2018 – 31 December 2020.
Main outcome measures: Rates of drug‐ and food‐related anaphylaxis, by age group; characteristics of cases of drug‐related anaphylaxis.
Results: A total of 4273 anaphylaxis episodes were reported (females: 2292 cases, 54%); the overall anaphylaxis rate was 31.9 episodes per 100 000 person‐years. The most frequently reported causes were foods (2659 cases, 62%); drugs were implicated in 533 cases (12%), insect venoms in 342 (8%), and other causes in 144 (4%). No deaths were recorded. The median age in cases of food‐related anaphylaxis was 17 years (IQR, 6–29 years), and 45 years (IQR, 30–60 years) in cases of drug‐related anaphylaxis. Hospitalisation was required by 1538 patients (36%) and intensive care by 111 (2.6%; 7% of people admitted to hospital). Antimicrobial drugs were implicated in 258 cases of drug‐related anaphylaxis (48%) and non‐steroidal anti‐inflammatory drugs in 85 cases (16%). Penicillin‐class agents were implicated in 143 cases of antimicrobial‐related anaphylaxis (56%), cephalosporins in 80 cases (31%).
Conclusion: Our review of notified cases of anaphylaxis in Victoria over two years provides insights into drug‐ and antimicrobial‐related anaphylaxis in non‐hospitalised people presenting to emergency departments.