Clinical record
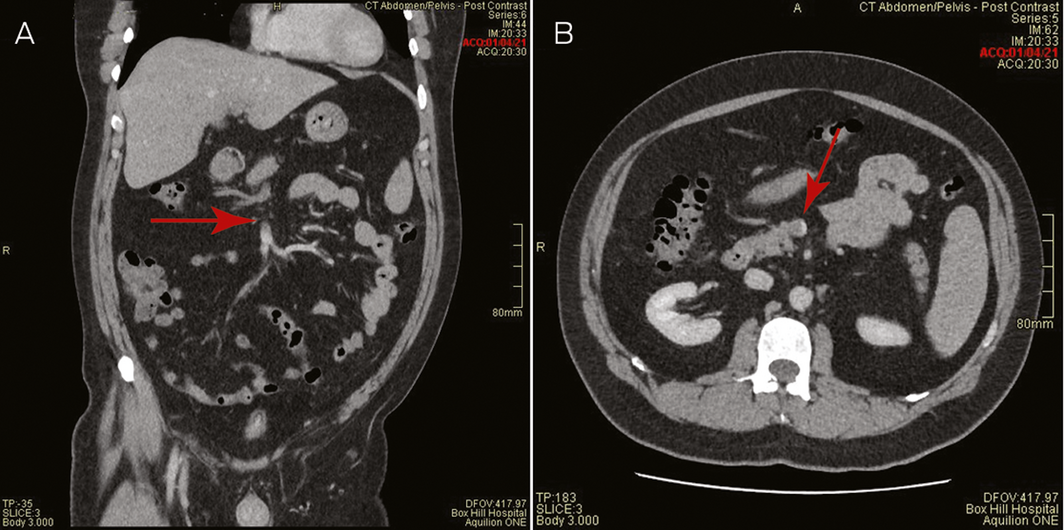
A 44‐year‐old male health care worker presented with fevers, fatigue and head “fogginess” with abdominal discomfort and increased bowel frequency 8 days after receiving his first dose of the COVID‐19 vaccine (ChAdOx1‐S [recombinant]) (AstraZeneca). He was previously well, with a past history of depression and was only taking escitalopram. He had no prior thrombosis or exposure to heparin. The low platelet count, 70 × 109/L (reference range, 150–400 × 109/L), and markedly elevated D‐dimer, 114 mg/L (upper limit of normal, 0.5 mg/L), with vague abdominal pains prompted a computed tomography (CT) venogram of the abdomen, which demonstrated thrombosis with complete occlusion of the portal and splenic veins and protrusion of a tongue of thrombus into the superior mesenteric vein (Box 1). CT venogram of the head did not show central venous sinus thrombosis.
The rest of the full blood count and the blood film showed no features of microangiopathic haemolytic anaemia. The prothrombin time, activated partial thromboplastin and fibrinogen levels, liver and renal function tests were all normal. Antiphospholipid antibodies were negative and a heterozygous prothrombin G20210A mutation was identified. Antibodies to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) nucleocapsid protein and the respiratory polymerase chain reaction (PCR) test for coronavirus disease 2019 (COVID‐19) were negative, ruling out active COVID‐19 infection as a potential thrombosis aetiology.
Antibodies to the heparin–platelet factor 4 (PF4) complex in the patient’s plasma was strongly positive (optical density, 1.94) by enzyme‐linked immunosorbent assay (ELISA)‐based test (Asserachrom HPIA IgG; Stago). Three functional assays, including serotonin release assay (SRA; Hidex 300 SL, Hydex), multiple electrode aggregometry (MEA; Roche Diagnostics) and flow cytometry (BD Fortessa, ETH Zürich; procoagulant assay) all detected heparin‐independent PF4 antibody complexes that activated donor platelets.1
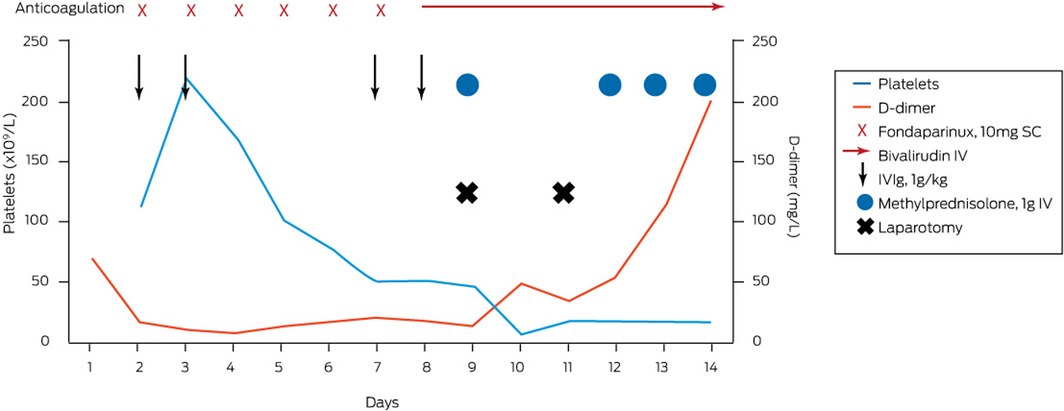
Immediate anticoagulation was started with the anti‐factor Xa agent fondaparinux 10 mg (weight, 105 kg) subcutaneously every 24 hours and intravenous immunoglobulin (1 g/kg) administered on days 2 and 3, and repeated on days 7 and 8 after admission. Despite achieving the anti‐factor Xa fondaparinux level 6 hours after receiving a dose of 0.94 U/mL (target range, 0.50–1.20 U/mL), the platelets remained between 6 × 109/L and 20 × 109/L for the initial 6 days. The patient subsequently developed an acute abdomen clinically and a repeat CT scan showed extension and occlusive thrombus into the superior mesenteric vein with venous outlet obstruction and bowel ischaemic features. He underwent an immediate laparotomy with resection of 1.8 m of ischaemic bowel. Contemporaneously, given clot extension had occurred on fondaparinux, anticoagulation was changed to thrombin inhibitor bivalirudin, which had a short half‐life (25 minutes) that allowed for titratable perioperative anticoagulation and an intravenous pulse of methylprednisolone 1 g administered to augment PF4 antibody immunosuppression.
The patient returned to the theatre 48 hours after the first laparotomy, where further compromised bowel was resected. Methylprednisolone 1 g daily was reinstituted for 4 days, with an immediate platelet peak to 385 × 109/L at completion of this 4‐day pulse. He was discharged after 34 days without further complications. He has transitioned to warfarin and continues to be well while monitored in the haematology outpatient clinic. Box 2 summarises the time course of treatment and response.
Discussion
In March 2021, Australia began the roll‐out of the COVID‐19 vaccine (ChAdOx1‐S [recombinant]) to combat the COVID‐19 pandemic. In Europe, where more than 20 million doses of this vaccine had been administered by mid‐March 2021, there were case reports of thrombosis at unusual sites associated with thrombocytopenia, which occurred at day 4–28 after vaccination and had a mortality rate of up to 25%, at an estimated rate of 1:100 000.2
In Australia, the COVID‐19 vaccine (ChAdOx1‐S [recombinant]) was initially offered to people working in high risk professions without age restrictions. By the end of March 2021, about 350 000 first doses of the COVID‐19 vaccine (ChAdOx1‐S [recombinant]) had been administered Australia‐wide.
This is the first reported case of thrombosis at an unusual site with thrombocytopenia following vaccination with the COVID‐19 vaccine (ChAdOx1‐S [recombinant]) in Australia. The temporal association, the detection of anti‐PF4 antibodies with platelet activation in the absence of heparin, which is neutralised at high dose heparin, is consistent with the most recent reports.3 This entity, currently labelled as vaccine‐induced thrombotic thrombocytopenia (VITT) — also known as thrombosis with thrombocytopenia syndrome (TTS) — has pathological similarity to autoimmune heparin‐induced thrombocytopenia but without prior heparin exposure. More evidence is needed to demonstrate if the serum of patients with VITT contains antibodies that can bind to PF4 independent of heparin following vaccination for COVID‐19. Furthermore, the mechanism responsible for profound platelet activation following vaccination with the COVID‐19 vaccine (ChAdOx1‐S [recombinant]), as evidenced by ELISA high optical densities, remains to be established.3 While the COVID‐19 vaccine (ChAdOx1‐S [recombinant]) is delivered via adenovirus vector, to the best of our knowledge, there are no reported VITT cases associated with mRNA COVID‐19 vaccines.
It is doctrine in the management of heparin‐induced thrombocytopenia that, in addition to immediate cessation of all heparins, a non‐heparin anticoagulant is commenced to prevent (further) thrombosis. Given the pathogenic similarities of VITT cases to heparin‐induced thrombocytopenia, we initially used fondaparinux, as our patient was clinically stable with normal renal function at presentation. It is unclear if the clinical deterioration in our patient, despite achieving favourable therapeutic fondaparinux drug level, was resultant of anti‐factor Xa drugs non‐efficacy and/or because of the severity of the venous outflow obstruction with compromised ischaemic small bowel. In principle, the surgical removal of any ischaemic tissue would be associated with clinical improvement.
The benefit of intravenous immunoglobulin remains debatable, but in vitro spiking experiments and observation of platelet increment after its administration suggest that there may be a role.3,4 It is possible that intravenous immunoglobulin displaces the binding of anti‐PF4 antibody complex to FcgammaRIIA (an Fc receptor for IgG) receptors on platelets.5 In our patient, it is difficult to ascribe a specific clinical and platelet recovery to intravenous immunoglobulin solely given the simultaneous timing of surgical removal of ischaemic intestine, the commencement of alternate anticoagulation with bivalirudin, and pulsed high dose steroids. It is noted that pulsed methylprednisolone was prescribed in the majority of recently reported cases.3
In line with evolving guidance documents, clinicians assessing patients who present with organ‐specific thrombotic symptoms 4–28 days following vaccination with the COVID‐19 vaccine (ChAdOx1‐S [recombinant]) should look for any combination of thrombocytopenia and elevated D‐dimer, and/or low fibrinogen, with a low threshold for requesting imaging of the appropriate organ — in particular, the brain central venous sinus and abdominal splanchnic venous systems — for thrombosis plus anti‐PF4 ELISA testing in consultation with haematology.
- Vaccine‐induced immune thrombotic thrombocytopenia (VITT) is rare but potentially life‐threatening.
- VITT should be considered when patients present at day 4–28 after vaccination with unusual site thrombosis: splanchnic and/or central venous sinus thrombosis, or thrombocytopenia (or falling platelets) and markedly elevated D‐dimer.
- Specific testing to detect anti‐platelet factor 4 (PF4) antibody is needed to support VITT.
- Treat with non‐heparin anticoagulation, intravenous immunoglobulin, and consider pulsed methylprednisolone. Avoid platelet transfusions.
Provenance: Not commissioned; externally peer reviewed.
- 1. Favaloro EJ, Mohammed S, Donikian D, et al. A multicentre assessment of contemporary laboratory assays for heparin induced thrombocytopenia. Pathology 2021; 53: 247–256.
- 2. Medicines and Healthcare products Regulatory Agency. MHRA issues new advice, concluding a possible link between COVID‐19 vaccine AstraZeneca and extremely rare, unlikely to occur blood clots [press release]. 7 Apr 2021. https://www.gov.uk/government/news/mhra-issues-new-advice-concluding-a-possible-link-between-covid-19-vaccine-astrazeneca-and-extremely-rare-unlikely-to-occur-blood-clots (viewed May 2021).
- 3. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med 2021; https://doi.org/10.1056/NEJMoa2104882 [Epub ahead of print].
- 4. Brodard J, Kremer Hovinga JA, Fontana P, et al. COVID‐19 patients often show high‐titer non‐platelet‐activating anti‐PF4/heparin IgG antibodies. J Thromb Haemost 2021; 19: 1294–1298.
- 5. Warkentin TE. High‐dose intravenous immunoglobulin for the treatment and prevention of heparin‐induced thrombocytopenia: a review. Expert Rev Hematol 2019; 12: 685–698.





No relevant disclosures.