The known: Low back pain is common among patients presenting to emergency departments. The short and long term efficacy of standard pharmaceutical interventions (paracetamol, non‐steroidal anti‐inflammatory drugs, opioids) is poor. Cannabidiol has anti‐inflammatory and analgesic properties, but its effect on acute low back pain has not been investigated.
The new: Single‐dose oral cannabidiol (400 mg) did not reduce pain scores in patients who presented to an emergency department with acute, non‐traumatic low back pain.
The implications: Cannabidiol does not reduce pain or hospital length of stay for people seeking help at a hospital emergency department for acute low back pain.
Low back pain is the most common musculoskeletal symptom among people visiting emergency departments, and it is one of the top five most frequent complaints in emergency medicine.1 A systematic review of 21 studies undertaken during 2000–2016 in 12 countries found that low back pain was consistently among the most frequent complaints in emergency medicine.2 Despite numerous studies and recommendations, a recent systematic review found that the utility of standard pharmacological treatments for acute back pain is limited.3 Accepted class II evidence indicates that patient education, superficial heat, paracetamol, non‐steroidal anti‐inflammatory drugs, and the limited use of skeletal muscle relaxants and opioids can be beneficial.1 It is reported that emergency physicians often choose opioid therapy, including 61% in a large American national sample4 and 70% in an Australian study.5 However, medical therapy for low back pain is of modest benefit.
Cannabidiol (CBD) is the major non‐psychotropic phytocannabinoid in Cannabis sativa (up to 40% of extracted alkaloids).6 Unlike most cannabinoids, including tetrahydrocannabinol (THC), CBD does not have psychomotor or cognitive effects, and its safety profile is good.6 CBD has attracted increasing medical interest because of its anxiolytic, anti‐inflammatory, anti‐emetic, anti‐epileptic, and anti‐psychotic effects.6,7,8,9 It has been approved in several countries for the treatment of neuropathic pain.10 The most frequently reported side effects of acute ingestion of CBD are dizziness or drowsiness, itching or rash, headache, abdominal discomfort, nausea, and vomiting or diarrhoea.9,11 Although CBD and its analogues may be beneficial for reducing pain caused by inflammation, its utility for treating acute low back pain has not been assessed.11
The objective of our study was to compare the analgesic effect and safety of single oral administration of CBD as an adjunct to standard care for patients who presented to an emergency department with acute, non‐traumatic low back pain, with those of a placebo.
Methods
The CANBACK trial was a single centre, randomised, double blinded, placebo‐controlled clinical trial. Adults with acute, non‐traumatic low back pain who presented to the Austin Hospital emergency department (Melbourne) during 21 May 2018 – 13 June 2019 were recruited. The Austin Hospital tertiary emergency department treats 90 000 patients each year, and offers a wide range of clinical services. The trial was registered with the Australian New Zealand Clinical Trials Registry on 4 April 2018 (ACTRN12618000487213).
Participants
Patients with acute low back pain were identified by the study investigators and clinical staff (medical and nursing) working in the triage and fast track areas of the emergency department, and were managed in the short stay unit. Participants were provided with a brief verbal description of the study and asked for written informed consent prior to enrolment. Analgesic medications used during the 24 hours preceding presentation, as reported by participants, were recorded in our data sheet.
We included people aged 18 years or more who presented with acute non‐traumatic low back pain — that is, pain and discomfort localised below the costal margin and above the inferior gluteal folds, as assessed by the treating physician — of less than 30 days’ duration. This definition included people with histories of low back pain. We excluded people who reported using cannabis or CBD in the preceding seven days, those with abnormal neurological examination findings (apart from subjective sensory changes), fever (exceeding 37.6°C), a history of malignancy, or a non‐musculoskeletal cause of back pain, and women who were pregnant (urinary β‐human chorionic gonadotropin testing of all women under 60 years of age).
Intervention
Most participants received standard emergency department drug treatment for low back pain; that is, 1000 mg paracetamol and 400 mg ibuprofen per os. However, treatment could be varied according to the patient’s recent drug use and their tolerance of non‐steroidal anti‐inflammatory drugs. Patients then received either the placebo (control group) or CBD (intervention group) (online Supporting Information). CBD (> 99.9% synthetic) and placebo were purchased as colour‐matched medications from GD Pharma (South Australia). The hospital pharmacy supplied 400 mg CBD in 4 mL medium chain triglyceride [MCT] oil) and placebo (4 mL MCT oil) as single oral syringe doses.
Pain scores — on a verbal numerical rating scale, ranging from 0 (no pain) to 10 (worst pain ever) — were recorded at triage, at 0, 30, 60, 90, and 120 minutes after drug administration, and at discharge from the emergency department.
Oxycodone (5 mg every 6 hours) was administered after 120 minutes if pain was not adequately managed, and the emergency department doctor could administer rescue analgesia (oxycodone) at any time if they deemed it clinically indicated.
Patients were monitored in the emergency department short stay unit for at least three hours after administration of the study agent and until pain was adequately controlled (ie, they could move safely, with oral analgesic medication as needed).
Data were collected on paper forms and transferred to a secured electronic Excel spreadsheet (Microsoft). Basic demographic information was extracted from the electronic hospital database (Cerner).
At least 48 hours after discharge from the emergency department, patients were followed up by phone to assess any delayed side effects and the need for further pain medication.
Dose selection
The selection of the 400 mg dose was based on safety and toxicology data for CBD in humans9 and on earlier studies of the therapeutic effects of CBD in children and adults.8,9,11 In one investigation,9 the effects of single oral 400 mg or 800 mg CBD doses administered together with intravenous fentanyl were assessed. The authors found that CBD was well tolerated, that there were no serious adverse events, and that its pharmacokinetic properties were not significantly affected by co‐administration with fentanyl; peak clinical effect was observed 1–2 hours after administration of 400 mg CBD, and plasma concentrations were similar at two hours for both dose levels.9 We therefore expected that 400 mg CBD would have a measurable effect.
Primary and secondary outcomes
The primary outcome measure was verbal numerical pain score (range, 0–10) two hours after administration of CBD or placebo. Secondary outcome measures were emergency department length of stay (including in the short stay unit), need for rescue analgesia, and any adverse event.
Sample size
We defined a 2‐point difference between mean pain scores for the two groups two hours after administration as being clinically significant.12 In order to detect a 2‐point difference in mean pain scores, we calculated that 47 patients in each group were required (α = 0.05, 2‐sided; power, 0.9). As the effect of CBD had not previously been assessed in an emergency department, this calculation was tentative; we therefore enrolled 50 patients in each group (100 participants in total).
Randomisation and allocation concealment
Consenting participants were randomised 1:1 to the intervention and control groups. We used an online permutation generator (twjc.co.uk/combinations.html) to generate permutation blocks (six patients per block); the blocks were then randomised (using the generator at random.org) to provide the final allocation list (maximum, 120 patients).
The study syringes were numbered consecutively by pharmacy staff and strictly allocated to patients as they were recruited. A list with the treatment break code was securely stored in the hospital pharmacy. The allocation sequence was generated and study medications prepared by a clinical trials pharmacist not involved in study design or recruitment. The content of each treatment pack (CBD or placebo) was known only to central hospital pharmacy staff. The treating clinicians, nursing staff, investigators, and participants were blinded to the study intervention.
Statistical analysis
The primary outcome of our intention‐to‐treat analysis was change in verbal numerical pain scores, summarised as means with 95% confidence interval (CIs). The statistical significance of differences between groups was assessed in unpaired t tests. Between‐group differences in non‐parametric variables were analysed in Mann–Whitney U tests; unpaired categorical data were assessed in χ2 tests, and paired continuous data in Wilcoxon matched pairs signed rank tests. Pain scores across time (every 30 minutes for two hours) were assessed by two‐way analysis of variance (ANOVA) by time after administration, with Šidák correction. Analyses were undertaken in SPSS for Windows 24.0; P < 0.05 was deemed statistically significant.
Ethics approval
Our trial was approved by the Austin Health Human Research Ethics Committee (reference, HREC/17/Austin/430).
Results
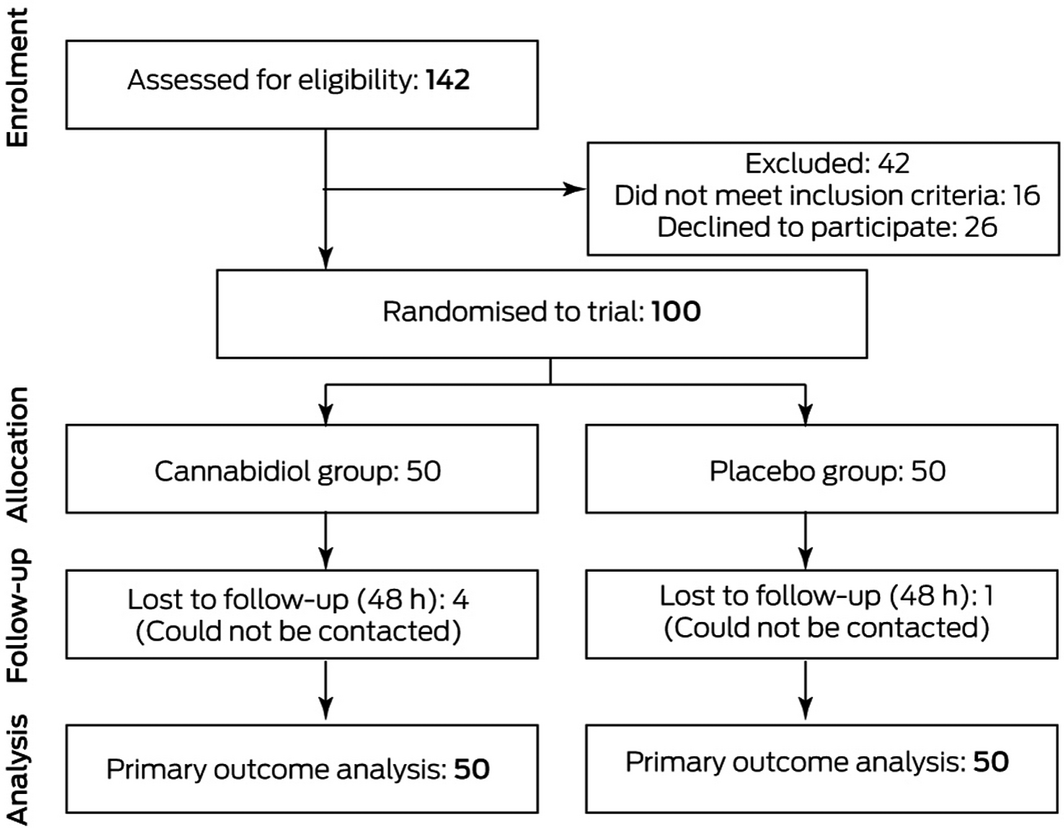
Of 142 patients assessed for eligibility, 100 were enrolled in the trial (Box 1). The median age of study participants was 47 years (interquartile range, IQR 34–60 years); 44 were women (Box 2). All study participants were fully ambulant, except for one participant in the placebo group who used a four‐wheel frame to assist with walking. No participant had previously taken CBD. Median time from initial emergency department analgesia to administration of the study agent was one hour (IQR, 0.5–1 h).
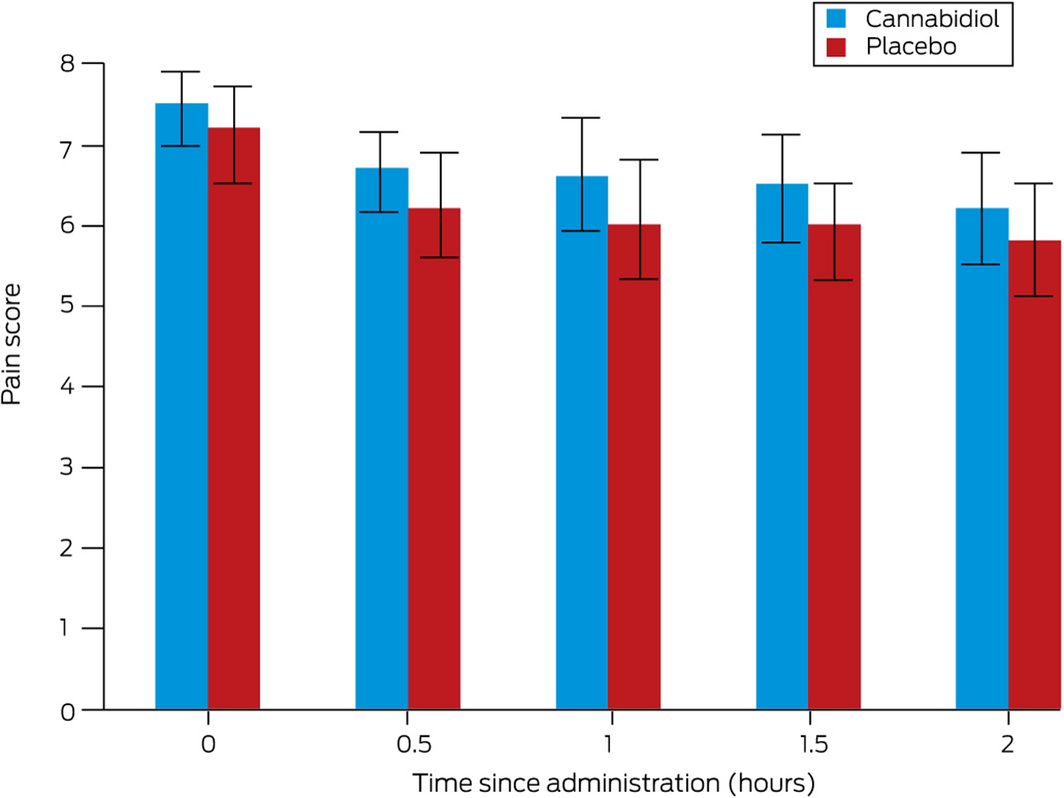
Complete pain scores data were available for all participants. Mean pain scores at two hours were similar for the CBD (6.2 points; 95% CI, 5.5–6.9 points) and placebo groups (5.8 points; 95% CI, 5.1–6.6 points; absolute difference, –0.3 points; 95% CI, –1.3 to 0.6 points) (Box 3). Two‐way ANOVA identified no difference between the cannabidiol and placebo groups at any time point (Supporting Information). The median length of emergency department stay was similar for the CBD (9.0 hours; IQR, 7.4–12 hours) and placebo groups (8.5 hours; IQR, 6.5–21 hours).
The amounts of oxycodone received during the four hours preceding or the four hours after administration of CBD or placebo were similar (Box 4), as were the median amounts of paracetamol (CBD and placebo groups: each 1000 mg; IQR, 1000–1000 mg) and ibuprofen (CBD and placebo groups: each 400 mg; IQR, 400–400 mg) received during the four hours after administration of the test agent. Apart from paracetamol, non‐steroidal anti‐inflammatory drugs (NSAIDs) and opioid medications were the agents most frequently used during the 24 hours before presenting to the emergency department (Box 5).
Side effects were similar for the two groups; the most frequent was sedation, both three hours (CBD, 8 participants; placebo, 7 participants) and 48 hours after administration of the study agent (CBD, 6 participants; placebo, 3 participants) (Box 6).
One participant presented to the emergency department again the following day (with fever); they were subsequently diagnosed with an epidural abscess requiring intervention. This event was deemed unrelated to the study medication (placebo).
At the 48‐hour follow‐up, participants were asked whether they wished to receive the same medication combination if they again presented to the emergency department with acute low back pain; 28 participants in the CBD group and 26 in the placebo group replied that they would. Five patients could not be contacted for the 48‐hour follow‐up, despite several attempts (CBD group, four; placebo group, one).
Discussion
The CANBACK trial was novel in that we investigated treating patients with one form of acute pain with a single high oral dose of CBD alone (ie, without THC). We found no evidence of benefit for patients with acute low back pain; the CBD and placebo groups did not differ with respect to hospital length of stay, adverse effects, and additional opioid medication use. Given its high cost, this is an important finding for patients who may consider requesting Therapeutic Goods Administration (TGA) approval for access to CBD.
A recent systematic review of cannabinoid medications (THC‐containing preparations) as analgesics for managing acute post‐surgical pain found that they were ineffective; however, the review did not find any trials in which CBD was used.11 A larger meta‐analysis that included randomised controlled trials and observational studies of cannabis and cannabinoid products for treating chronic non‐cancer pain similarly concluded that they were ineffective for this purpose.13
Five older randomised controlled trials of cannabinoids for treating chronic non‐cancer pain included CBD, either alone or in combination with THC; all were double‐blinded, and all but one employed crossover designs.14,15,16,17,18 In all five trials, a combination of CBD and THC achieved significantly greater pain reduction than placebo.14,15,16,17,18 Of the two trials that compared THC, CBD, and a combination of the two with placebo,17,18 one found significantly greater reduction of pain with each of CBD, THC, and CBD/THC than with placebo.18
Although these studies reported significant reductions in pain, the sample sizes were all relatively small (range, 40–124 participants). Side effects (eg, sedation) were more frequent with CBD/THC than with CBD alone, and the number of patients who withdrew from the trials was substantial. The investigators used different routes of administration (sublingual, oral sprays, oral), and CBD doses ranged from 2.5 mg to 120 mg. Given the wide range of study conditions, it would be inappropriate to generalise their findings or to apply them in clinical practice.
Implications for clinicians and policymakers
Given increasing attention worldwide to the problem of opioid misuse, people are seeking alternative pain control methods for conditions such as back pain. The Australian TGA has provided guidance documents for using medicinal cannabis in patients with chemotherapy‐induced nausea and vomiting, refractory paediatric epilepsy, palliative care indications, cancer pain, neuropathic pain, spasticity in neurological conditions, or anorexia and wasting associated with chronic illness, such as cancer.19 With the gradual legalisation of medicinal and recreational cannabis use in Australia and other countries, physicians must expect to see increasing numbers of patients taking cannabis products, whether prescribed by physicians or not. It is imperative that the medical utility of CBD and other cannabis products, their side effects, and how these products interact with other medications be investigated in well designed studies.
Limitations
Although the verbal numerical pain scale is both widely used and accepted, this tool lacks objective features; adjunctive tools, such as the functional activity score,20 could be used in future studies. Analgesic medications taken prior to arrival in the emergency department may have affected pain scores. However, pain scores were similar in the intervention and control groups, both at triage and during the two hours after administering the study agent. Finally, we used a single oral dose approach rather than repeated administration of CBD, which may have different effects.
Conclusion
The CANBACK trial was the largest clinical investigation of CBD for treating people with acute low back pain, was one of the few to examine the effect of CBD alone (without THC) on acute pain, and was undertaken in an environment that reflected clinical management of acute back pain in emergency departments. We found that CBD was not superior to placebo as an adjunct medication for treating low back pain in this setting.
Data sharing statement
All data are available upon request to the study investigators.
Box 1 – CONSORT flow diagram of the CANBACK trial of oral cannabidiol for people presenting to the emergency department with acute low back pain
Box 2 – Baseline demographic and clinical characteristics of participants in the CANBACK trial
|
|
Cannabidiol group |
Placebo group |
|||||||||||||
|
|
|||||||||||||||
|
Number of participants |
50 |
50 |
|||||||||||||
|
Age (years), median (IQR) |
52 (41–59) |
42 (31–60) |
|||||||||||||
|
Sex (women) |
22 |
22 |
|||||||||||||
|
Pain score at triage, mean (SD) |
7.1 (2.5) |
7.4 (2.1) |
|||||||||||||
|
Pain duration before presentation (days), median (IQR) |
1 (1–4) |
1 (1–4) |
|||||||||||||
|
Paracetamol in emergency department prior to study agent |
46 |
45 |
|||||||||||||
|
Non‐steroidal anti‐inflammatory drug in emergency department prior to study drug |
40 |
42 |
|||||||||||||
|
Prior spinal surgery |
1 |
3 |
|||||||||||||
|
Chronic back pain (recurrent exacerbation of back pain) |
10 |
11 |
|||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. |
|||||||||||||||
Box 3 – Mean verbal numerical pain scores (with 95% confidence intervals) during the two hours after administration of cannabidiol or placebo
Box 4 – Oxycodone use before and after the administration of cannabidiol or placebo as trial agent
|
|
Cannabidiol group |
Placebo group |
|||||||||||||
|
|
|||||||||||||||
|
4 hours before trial drug (mg), median (IQR) |
5 (0–15) |
5 (0–5) |
|||||||||||||
|
Number of patients who received oxycodone |
32 |
24 |
|||||||||||||
|
Total group use (mg) |
235 |
165 |
|||||||||||||
|
4 hours after trial drug (mg), median (IQR) |
5 (0–10) |
5 (0–10) |
|||||||||||||
|
Number of patients who received oxycodone |
31 |
27 |
|||||||||||||
|
Total group use (mg) |
230 |
215 |
|||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. |
|||||||||||||||
Box 5 – Medications taken by study participants in the 24 hours before they presented to the emergency department
|
|
Cannabidiol group |
Placebo group |
|||||||||||||
|
Medication |
Number of participants |
Median amount (IQR) |
Number of participants |
Median amount (IQR) |
|||||||||||
|
|
|||||||||||||||
|
Opioids |
|
|
|
|
|||||||||||
|
Oxycodone |
4 |
5 mg (5–10 mg) |
2 |
5 mg (5–10 mg) |
|||||||||||
|
Fentanyl (intravenous) |
1 |
75 μg |
1 |
50 μg |
|||||||||||
|
Fentanyl transdermal |
1 |
75 μg/hr |
0 |
— |
|||||||||||
|
Codeine |
2 |
60 mg (60–210 mg) |
3 |
60 mg (45–75 mg) |
|||||||||||
|
Tapentadol |
0 |
— |
1 |
100 mg |
|||||||||||
|
Morphine (intravenous) |
0 |
— |
2 |
5 mg (5–10 mg) |
|||||||||||
|
Extended‐release morphine |
0 |
— |
1 |
30 mg |
|||||||||||
|
Tramadol |
1 |
100 mg |
2 |
125 mg (100–150 mg) |
|||||||||||
|
Non‐steroidal anti‐inflammatory drug |
|
|
|
|
|||||||||||
|
Ibuprofen |
14 |
400 mg (400–700 mg) |
18 |
600 mg (400–800 mg) |
|||||||||||
|
Meloxicam |
2 |
15 mg (7.5–15 mg) |
2 |
15 mg (15–15 mg) |
|||||||||||
|
Celecoxib |
1 |
100 mg |
0 |
— |
|||||||||||
|
Naproxen |
0 |
— |
3 |
1.75 g (0.5–3.0 g) |
|||||||||||
|
Indomethacin |
0 |
— |
2 |
50 mg (50–100 mg) |
|||||||||||
|
Diclofenac |
5 |
50 mg (50–50 mg) |
0 |
— |
|||||||||||
|
Other |
|
|
|
|
|||||||||||
|
Paracetamol |
29 |
1 g (1–2 g) |
25 |
1 g (1–2 g) |
|||||||||||
|
Pregabalin |
2 |
75 mg (75–300 mg) |
3 |
75 mg (20–150 mg) |
|||||||||||
|
Gabapentin |
0 |
— |
1 |
300 mg |
|||||||||||
|
Methoxyflurane |
3 |
1 × 3 mL inhaler |
2 |
1 × 3 mL inhaler |
|||||||||||
|
Amitriptyline |
1 |
25 mg |
0 |
— |
|||||||||||
|
Diazepam |
3 |
5 mg (4–10 mg) |
4 |
10 mg (6–25 mg) |
|||||||||||
|
Orphenadrine |
1 |
100 mg |
0 |
— |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. |
|||||||||||||||
Box 6 – Side effects reported by CANBACK trial participants three hours after receiving study agent and at 48‐hour follow‐up
|
Side effect |
Cannabidiol group |
Placebo group |
|||||||||||||
|
|
|||||||||||||||
|
Sedation |
|
|
|||||||||||||
|
3 hours |
8 |
7 |
|||||||||||||
|
48 hours |
6 |
3 |
|||||||||||||
|
Diarrhoea |
|
|
|||||||||||||
|
3 hours |
0 |
0 |
|||||||||||||
|
48 hours |
1 |
2 |
|||||||||||||
|
Nausea |
|
|
|||||||||||||
|
3 hours |
5 |
4 |
|||||||||||||
|
48 hours |
5 |
10 |
|||||||||||||
|
Vomiting |
|
|
|||||||||||||
|
3 hours |
0 |
0 |
|||||||||||||
|
48 hours |
1 |
4 |
|||||||||||||
|
Headache |
|
|
|||||||||||||
|
3 hours |
0 |
0 |
|||||||||||||
|
48 hours |
4 |
4 |
|||||||||||||
|
Other |
|
|
|||||||||||||
|
3 hours |
Lightheadedness 1 |
Dizziness, 1 |
|||||||||||||
|
48 hours |
Constipation, 3 |
Constipation, 1 |
|||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 21 May 2020, accepted 20 November 2020
- Bronwyn Bebee1
- David M Taylor1,2
- Elyssia Bourke1
- Kimberley Pollack1
- Lian Foster1
- Michael Ching1
- Anselm Wong1,2,3,4
- 1 Austin Health, Melbourne, VIC
- 2 University of Melbourne, Melbourne, VIC
- 3 Victorian Poisons Information Centre, Austin Hospital, Melbourne, VIC
- 4 Monash University, Melbourne, VIC
We thank the staff of the Austin Hospital emergency department for their assistance with the study, and the Robert C. Bulley Charitable Fund and the Austin Medical Research Foundation for funding the CANBACK trial. Anselm Wong holds a National Health and Medical Research Council fellowship (1159907).
No relevant disclosures.
- 1. Borczuk P. An evidence-based approach to the evaluation and treatment of low back pain in the emergency department. Emerg Med Pract 2013; 15: 1–23.
- 2. Edwards J, Hayden J, Asbridge M, et al. Prevalence of low back pain in emergency settings: a systematic review and meta-analysis. BMC Musculoskelet Disord 2017; 18: 143.
- 3. Chou R, Deyo R, Friedly J, et al. Systematic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Internal Med 2017; 166: 480–493.
- 4. Edlow JA. Managing nontraumatic acute back pain. Ann Emerg Med 2015; 66: 148–513.
- 5. Ferreira GE, Machado GC, Shaheed CA, et al. Management of low back pain in Australian emergency departments. BMJ Qual Saf 2019; 28: 826–834.
- 6. Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol: recent advances. Chem Biodivers 2007; 4: 1678–1692.
- 7. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol; a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2017; 2: 139–154.
- 8. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017; 376: 2011.
- 9. Manini AF, Yiannoulos G, Bergamaschi MM, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med 2015; 9: 204–210.
- 10. Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics 2009; 6: 713–737.
- 11. Stevens AJ, Higgins MD. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand 2017; 61: 268–280.
- 12. Kelly AM. Setting the benchmark for research in the management of acute pain in the emergency departments. Emerg Med 2001; 13: 57–60.
- 13. Stockings E, Campbell G, Hall W, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018; 159: 1932–1954.
- 14. Blake D, Robson P, Ho M, et al. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology 2006; 45: 50–52.
- 15. Nurmikko TJ, Serpell MG, Hoggart B, et al. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo‐controlled clinical trial. Pain 2007; 133: 210–220.
- 16. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain 2004; 112: 299–306.
- 17. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 “N of 1” studies. Anaesthesia 2004; 59: 440–452.
- 18. Wade DT, Robson P, House H, et al. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil 2003; 17: 21–29.
- 19. Therapeutic Goods Administration. Medicinal cannabis. Updated 25 Sept 2020. https://www.tga.gov.au/medicinal-cannabis (viewed Mar 2021).
- 20. Tong YG, Konstantatos AH, Yan C, Ling C. Improving pain management through addition of the functional activity score. Australian Journal of Advanced Nursing 2018; 35: 52–60.





Abstract
Objective: To assess the analgesic efficacy and safety of single‐dose oral cannabidiol (CBD) as an adjunct to standard care for patients presenting to an emergency department with acute low back pain.
Design: Randomised, double blinded, placebo‐controlled clinical trial.
Setting: The tertiary emergency department of Austin Hospital, Melbourne.
Participants: Patients who presented with acute, non‐traumatic low back pain between 21 May 2018 and 13 June 2019.
Intervention: One hundred eligible patients were randomised to receiving 400 mg CBD or placebo in addition to standard emergency department analgesic medication.
Main outcome measures: Pain score two hours after administration of study agent, on a verbal numerical pain scale (range, 0‒10). Secondary outcomes were length of stay, need for rescue analgesia, and adverse events.
Results: The median age of the 100 participants was 47 years (IQR, 34‒60 years); 44 were women. Mean pain scores at two hours were similar for the CBD (6.2 points; 95% CI, 5.5–6.9 points) and placebo groups (5.8 points; 95% CI, 5.1–6.6 points; absolute difference, –0.3 points; 95% CI, –1.3 to 0.6 points). The median length of stay was 9.0 hours (IQR, 7.4‒12 hours) for the CBD group and 8.5 hours (IQR, 6.5‒21 hours) for the placebo group. Oxycodone use during the four hours preceding and the four hours after receiving CBD or placebo was similar for the two groups, as were reported side effects.
Conclusion: CBD was not superior to placebo as an adjunct medication for relieving acute non‐traumatic low back pain in the emergency department.
Trial registration: Australian New Zealand Clinical Trials Registry, ACTRN12618000487213 (prospective).