This consensus statement is applicable to specialists, general medical practitioners, nurses, health coordinators and administrators involved in the care of adult patients with hepatocellular carcinoma (HCC).
These recommendations summarise the complete document, available at https://www.gesa.org.au/resources/hepatocellular-carcinoma-hcc-management-consensus/.
Methodology
Recommendations were graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE).1 The quality of the evidence was classified as high, moderate, low, or very low, and the strength of recommendation was classified as either strong or weak.
This consensus statement was developed with the principles outlined by the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument.2
Consensus was defined as a greater than 80% agreement by experts. The modified Delphi process was used to determine consensus and comprised two face‐to‐face meetings and three rounds of online questionnaires.3 The complete list of recommendations and the level of agreement are shown in Box 1.
Epidemiology and surveillance
Globally, hepatocellular carcinoma is a leading cause of cancer death and the seventh most common cancer.4 In Australia, the incidence has increased markedly from 1982 (1.38/100 000) to 2014 (4.96/100 000)5 and within Australia there is significant regional variation in incidence. Although mortality rates of many cancers have plateaued, cancer death due to HCC is rising,6 and despite improvements in treatment, the overall 5‐year survival in Australia is about 20%.7
HCC usually develops in the setting of chronic liver disease, and cirrhosis is present in 85–90% of affected individuals. Male incidence is three to four times that of females7,8 and the major aetiologies for HCC are shared with the causes of cirrhosis (hepatitis C virus [HCV] infection, 41%; alcohol‐related liver disease, 39%; hepatitis B virus [HBV] infection, 22%; and fatty liver disease, 14%).8 HBV infection is more common in culturally and linguistically diverse populations, and in Australia, more than 50% of patients with HCC were born overseas.8
Incidence of HCC in Australian Indigenous populations
Indigenous Australians account for 3.3% of the population, yet are disproportionately affected by HCC, with 2.4‐fold higher rates of diagnosis and mortality compared with non‐Indigenous populations.7 Inequalities in health service access with resulting late presentation, greater incidence of risk factors for liver disease, socio‐economic disadvantage and geographical remoteness of Indigenous communities in many parts of Australia contribute to a greater burden of HCC incidence and mortality.9 Chronic HBV and HCV infection prevalence is estimated at 6% and 2.9% respectively among Indigenous Australians in the Northern Territory.10 Of concern, among Indigenous Australians, only 14% of HCC is detected through surveillance programs, and the median survival time is markedly lower in Indigenous compared with non‐Indigenous Australians (64 v 172 days).11
Target populations for HCC surveillance in Australia
Several target populations for surveillance have been defined based on decision analyses that show surveillance becomes cost‐effective when the incidence of HCC in these at‐risk populations approaches certain thresholds.12 The single most significant risk factor for developing HCC is cirrhosis.13 However, HCC risk is sufficiently high in some non‐cirrhotic patients with hepatitis B to justify surveillance11 (Box 2).
Surveillance modalities available for detecting HCC in Australia
Liver ultrasound
Liver ultrasound, the primary tool recommended for HCC surveillance, is widely available, non‐invasive, comparatively inexpensive, and has Australian Medicare Benefit Schedule (MBS) reimbursement. The sensitivity of ultrasound used for HCC surveillance varies between 60% and 90%, with specificity generally above 90%. Computed tomography (CT) and magnetic resonance imaging (MRI) are not suitable routine surveillance modalities for HCC.
The optimal HCC surveillance interval reflects the tumour doubling time of 4–8 months. Several international cohort studies have shown that HCC surveillance every 6 months is cost‐effective and improves survival.14
Serum marker: α‐fetoprotein
Serum α‐fetoprotein may increase the sensitivity of surveillance but can be associated with false‐positive results. As α‐fetoprotein improves earlier detection of HCC compared with ultrasound alone, we recommend surveillance with a combination of ultrasound and serum α‐fetoprotein levels every 6 months.
Populations not requiring HCC surveillance
The expectation with HCC surveillance is that it will reduce mortality by allowing patients to access curative therapy. Benefit is not seen if the patient is expected to die of progressive liver failure or other comorbid conditions before receiving treatment. The survival benefit of HCC surveillance appears to be restricted to patients with Child–Pugh class A and early class B cirrhosis and with Child–Pugh class C disease who are candidates for liver transplantation.15
Non‐alcoholic fatty liver disease and its association with HCC incidence
Non‐alcoholic fatty liver disease patients with cirrhosis should undergo surveillance. Meta‐analyses have shown that obesity may increase the relative risk of HCC by 1.5‐ to four‐fold.16,17 Diabetes increases the relative risk of developing HCC by two‐ to 2.5‐fold and increases HCC‐associated mortality by 1.6‐fold.18,19 In a large retrospective study, the relative risk of developing HCC was seven‐fold that of controls. Most cases occurred in cirrhotic individuals, with the relative incidence in non‐cirrhotic subjects not reaching the accepted threshold to justify surveillance.20 Therefore, surveillance for HCC in non‐cirrhotic patients with non‐alcoholic fatty liver disease is not supported by current evidence.
Diagnosis and staging
Unlike other malignant neoplasms, the diagnosis of HCC may be made principally on radiological criteria. The Liver Imaging Reporting and Data System (LI‐RADS)21 provides a framework for reporting and criteria for HCC diagnosis.
In individuals with high pre‐test probability for HCC (ie, with cirrhosis or HBV infection) the diagnosis of HCC can be confidently made when non‐rim arterial phase hyperenhancement (APHE) and portal venous or delayed phase washout is present.22 In patients with a lower risk of HCC (eg, non‐cirrhotic patients and those without hepatitis B) or when imaging characteristics are concerning but not typical for HCC and malignancy is suspected, targeted biopsy with histological confirmation is indicated (Box 3).
Imaging criteria for diagnosis of HCC
Computed tomography
Multiphase CT with pre‐contrast and three post‐contrast phases (late hepatic arterial, portal venous, and delayed) is the optimal imaging protocol for HCC diagnosis. The appearances of non‐rim APHE together with washout during the portal or delayed phase confer a specificity approaching 100%. The sensitivity of these radiological findings is highly dependent on lesion size, with excellent sensitivity for lesions greater than 2 cm, modest sensitivity for lesions 1–2 cm, and poor sensitivity for lesions less than 1 cm in diameter.23
Magnetic resonance imaging
The principles of HCC diagnosis are similar for both MRI and CT. With the use of extracellular contrast agents, the features of non‐rim APHE and washout are highly suggestive of HCC in individuals at risk. Compared with multiphase CT, MRI offers additional imaging sequences that may provide supportive information for the diagnosis of HCC, including T2‐weighted sequences, diffusion‐weighted imaging and hepatobiliary phase imaging (when liver‐specific contrast agents are used).
Contrast‐enhanced ultrasound
Contrast‐enhanced ultrasound is not widely practised in Australia and requires skilled operators, but can be used to interrogate a suspicious lesion and has the advantage of avoiding ionising radiation and nephrotoxic contrast agents. As with cross‐sectional imaging modalities, such as CT and MRI, the imaging features of APHE and washout pertain to contrast‐enhanced ultrasound.
Choice of imaging modality
Several practical factors determine the choice between CT, MRI and contrast‐enhanced ultrasound. Patients who experience claustrophobia, who are unable to hold their breath sufficiently long or who have ascites (which may result in image artefacts) may be better imaged with CT. However, CT requires ionising radiation exposure and potential nephrotoxicity from intravenous contrast agents.24 In Australia, the choice between MRI and CT has been influenced by accessibility of the imaging techniques and MBS reimbursement. Contrast‐enhanced MRI liver scans (including the use of hepatobiliary‐specific contrast agents) for the purpose of diagnosis or staging of known or suspected HCC is funded by the MBS in Australia (one scan per 12‐month period).
Staging systems
Barcelona Clinic Liver Cancer staging system
The Barcelona Clinic Liver Cancer (BCLC) staging system is used and endorsed by international guidelines.22,25,26 It incorporates three clinical aspects: liver function, tumour biology and performance status, and offers treatment recommendations based on stage (Box 4). The BCLC staging system is the most commonly used system in multidisciplinary team meetings in Australia and is likely to remain the system of preference because of physician familiarity and ease of use.
Management
Management of HCC in Australia is to offer curative intent when possible while minimising exposure to risk with treatment. Staging of disease and determination of appropriate management through a multidisciplinary team are critical. A patient’s understanding of their disease and the clinician’s respect for patient choices are essential elements of HCC management. Managing clinicians are expected to offer evidence‐based treatment options.
The multidisciplinary team approach
Multidisciplinary team discussion is recommended for every patient diagnosed and being managed with HCC. Multidisciplinary team care is being increasingly practised in cancer care services in Australia, the United States and Europe.27 This type of care has several benefits, including improvement in staging and diagnosis accuracy, increased treatment rates, reduction in time to treatment after diagnosis, and increased adherence to clinical guidelines.28 Several retrospective cohort studies show an improvement in the overall survival of patients with HCC managed through a multidisciplinary team.29
Surgical therapies
Surgical resection
Liver resection is indicated and recommended for HCC in patients in whom the tumour is confined to the liver and can be completely removed, while leaving a sufficient liver remnant in terms of both quantity and quality to preserve life. Therefore, assessment of patients for potential liver resection for HCC requires evaluation of the patient, liver function, portal hypertension and tumour characteristics.25 Overall survival after liver resection for HCC depends on the risk of developing liver failure, succumbing to non‐liver‐related complications of surgery, or developing recurrent HCC.
Liver transplantation
Liver transplantation is a definitive treatment option for patients with early stage HCC, as it eliminates both the tumour and the associated liver disease.30 In Australia and New Zealand, the 5‐year overall survival among liver transplant recipients for HCC is 75%, similar to overseas experience.31
Appropriate patient selection for liver transplantation is critical to both achieving optimal outcomes and appropriate donor organ utilisation. Eligibility for entry to the liver transplantation waiting list should be based on an expected 5‐year survival rate greater than 50%. The University of California San Francisco (UCSF) criteria32 provides the current framework for patient selection in Australia (Box 5).31,32,33,34 Patients within UCSF criteria have survival rates after liver transplantation of 90% and 75.2% at 1 and 5 years respectively, compared with a 50% 1‐year survival for patients with tumours exceeding these limits.32 An alternative system, Metroticket 2.0, incorporating α‐fetoprotein and tumour characteristics under review by the Australasian transplant societies may broaden patient selection without adverse impact on patient outcomes.34
Locoregional therapies
More than 75% of patients with early stage (BCLC‐A) HCC are not suitable for either surgical resection or liver transplantation because of underlying severity of liver disease, clinically significant portal hypertension, significant comorbidity, or age.35 For these patients, locoregional therapies, with image‐guided percutaneous tumour ablation and/or image‐guided transcatheter tumour therapy should be considered. Ablative therapies are generally restricted to small number of lesions (three or fewer), while non‐ablative therapies may be used when multiple lesions are present.
Ablative therapies
Ablative therapies for HCC involve treatment of a lesion with the intent of local disease elimination.
Percutaneous tumour ablative therapies
Percutaneous tumour ablation under imaging guidance is an important and widely accepted treatment option for patients with early stage HCC. The two common methods used to induce tumour necrosis are temperature alteration (eg, radiofrequency ablation and microwave ablation) and chemical injection (most commonly percutaneous ethanol injection). Radiofrequency ablation is the most widely recommended first‐line ablation technique for patients not suitable for surgery.36 Microwave ablation is a relatively recent and increasingly popular thermal ablative therapy37 that has the advantages of producing wider and more predictable ablation volumes, resulting in high complete ablation rates, and the ability to simultaneously treat multiple lesions36,37 and potentially treat larger lesions more effectively. Ablation is effective in early stage (BCLC‐0) HCC involving a solitary small nodule (< 2 cm), achieving near 100% complete necrosis and with overall survival similar to that of surgery.38
Non‐ablative therapies
Non‐ablative therapies for HCC involve treatment of a lesion with the intent of local disease control. Although small lesions may be cured, typically lesions recur, requiring further treatment. Transarterial chemoembolisation (TACE) is the most common non‐ablative therapy for HCC.
Transarterial chemoembolisation
TACE involves injection of chemotherapy and embolic material into hepatic artery branches supplying a tumour and is considered first‐line therapy for patients with BCLC‐B HCC.25,39 It is regarded as a non‐curative procedure in this stage of cancer.
Meta‐analyses have demonstrated that patients treated with TACE derive a significant improvement in overall survival compared with bland embolisation or best supportive care.40 An overall response rate of about 50% and a disease control rate of 75–80% can be expected with TACE therapy.41
Selective internal radiation therapy
Selective internal radiation therapy (SIRT) involves Yttrium‐90 microspheres injection into the hepatic arteries supplying the tumour. Radioembolisation has been used instead of TACE for patients with BCLC‐B disease, as an alternative to sorafenib for patients with BCLC‐C disease (including portal vein invasion), and for bridging or downstaging to liver transplantation. However, the evidence base justifying the use of SIRT is less advanced than that for other therapies. Three randomised controlled studies failed to demonstrate superiority of SIRT over sorafenib. This has contributed to the uncertainty around the precise role of SIRT in HCC management.42,43,44 However, SIRT can be considered in select patients with intermediate or locally advanced HCC.
Other locoregional therapies
Stereotactic external‐beam radiation therapy
Stereotactic external‐beam radiation therapy (SBRT) is emerging as a potential option for early stage disease not amenable to surgical or percutaneous ablative therapies. A systematic review of SBRT for early stage HCC incorporating 16 studies (973 patients, 1034 lesions) reported a mean weighted local control of 94% and 93% at 1 and 3 years respectively.45 For more advanced disease, a small randomised study reported improved overall survival with upfront TACE and SBRT compared with sorafenib in the setting of macrovascular invasion (median, 55 weeks v 43 weeks; P = 0.04).46 Further prospective randomised studies are required before SBRT can be recommended for routine use.
Systemic therapies
Systemic therapies are indicated in patients with advanced HCC, with vascular invasion and/or extrahepatic disease, or in patients with unresectable HCC when locoregional therapies have failed to control disease or cannot be delivered. Systemic therapy is restricted to patients with preserved liver function, non‐cirrhotic patients, or those with Child–Pugh class A cirrhosis.
There are currently three first‐line therapies for HCC: sorafenib, lenvatinib and combination atezolizumab and bevacizumab. Sorafenib was the first therapy to show an increase in median overall survival from 7.9 to 10.7 months (hazard ratio [HR], 0.70; 95% CI, 0.55–0.87).47 In a subsequent trial comparing lenvatinib with sorafenib, lenvatinib was non‐inferior to sorafenib The median overall survival was 13.6 months (95% CI, 12.1–14.9) with lenvatinib and 12.3 months (95% CI, 10.4–13.9) with sorafenib (HR, 0.92; 95% CI, 0.79–1.06).48 Progression‐free survival was significantly higher in patients receiving lenvatinib (median, 7.4 months; 95% CI, 6.9–8.8) compared with sorafenib (median, 3.7 months [95% CI, 3.6–4.6]; HR, 0.66 [95% CI, 0.57–0.77]). The combination of atezolizumab (programmed death‐ligand 1 antibody) and bevacizumab (antivascular endothelial growth factor) has shown significant improvement in progression‐free survival (median, 6.8 months; 95% CI, 5.7–8.3) compared with sorafenib (median, 4.3 months; 95% CI, 4.0–5.6) and is the most recently approved therapy in Australia.49
There are no clear benefits to continuing first‐line therapies in patients with radiological or clinical disease progression. Second‐line treatments approved by the Therapeutic Goods Administration include two oral targeted therapies (regorafenib and cabozantinib) and nivolumab (immune checkpoint inhibitor) administered by intravenous infusion.
Systemic therapies for advanced HCC are evolving rapidly, and it is likely that the standard of care will change in the near future.
Supportive care
Many patients either present with incurable disease or progress after failed attempts at curative therapy. Patients with incurable HCC should be introduced to supportive care services early in their management (Box 6). Patients presenting with advanced disease (15–20% of cases) have a median survival of less than 3–4 months. The estimated 1‐year survival rate of patients with BCLC‐D disease is less than 11%.50 In addition to cancer‐related complications, clinical deterioration may also be related to the underlying liver disease, which has unique clinical challenges. Symptom relief and psychosocial support may be most effectively achieved through the co‐management of patients by hepatologists and supportive or palliative care services.
For patients with BCLC‐D disease, management should occur in conjunction with supportive care services and should focus on symptom relief. Pain is a common feature of HCC and can result from both the disease and its treatment. The analgesic choice must take into consideration the severity of liver disease, portosystemic shunting, low circulating albumin (changing bioavailability), risk of return to opioid dependence, current medications, and clinical features, including hepatic encephalopathy and hepatorenal syndrome.51 The World Health Organization analgesic ladder provides an accepted treatment algorithm for analgesia use in all cancers, including HCC.
Conclusion
The management of HCC requires a team of experts delivering multiple therapeutic modalities while balancing the issues of pre‐existent liver disease and risk of hepatic decompensation. Overall, mortality remains high despite all the recent advances in locoregional and systemic therapies. A priority for Australia, is to recognise patients at risk of HCC and to institute surveillance strategies at a time when the HCC is curable.
Box 1 – Recommendations of the hepatocellular carcinoma (HCC) consensus statement
|
No. |
Consensus recommendation |
GRADE quality of evidence* |
Level of agreement |
||||||||||||
|
|
|||||||||||||||
|
1 |
HCC surveillance should be offered to all patients with cirrhosis if they are suitable and willing to receive treatment |
C1 |
48 (100%) |
||||||||||||
|
2 |
HCC surveillance should be undertaken in non‐cirrhotic individuals with chronic HBV infection who are at increased risk of HCC |
C1 |
46 (97.8%) |
||||||||||||
|
3 |
Surveillance for HCC should be undertaken using liver ultrasound every 6 months |
B1 |
49 (98.0%) |
||||||||||||
|
4 |
Combining α‐fetoprotein testing with liver ultrasound may be considered for HCC surveillance |
C2 |
45 (88.9%) |
||||||||||||
|
5 |
Antiviral therapy for HCV infection may be offered to patients with HCC who have undergone surgical or locoregional treatment with curative intent |
B2 |
40 (92.5%) |
||||||||||||
|
6 |
Patients with HCV infection‐related cirrhosis who achieve sustained virological response and undergo curative therapy for their HCC require ongoing surveillance |
B1 |
46 (95.7%) |
||||||||||||
|
7 |
HCC surveillance in non‐cirrhotic patients can be considered in select patient populations |
C2 |
43 (90.7%) |
||||||||||||
|
8 |
In the setting of cirrhosis, imaging diagnosis of HCC should rely on standardised criteria, based on evidence and validated in clinical practice |
B1 |
50 (98.0%) |
||||||||||||
|
9 |
Multiphase CT or MRI is the recommended investigation for lesions suspicious for HCC |
A1 |
50 (98.0%) |
||||||||||||
|
10 |
For indeterminate lesions > 10 mm diameter in cirrhotic livers, either targeted liver biopsy or repeat interval imaging or an alternative imaging modality is required for diagnosis |
B1 |
45 (97.8%) |
||||||||||||
|
11 |
It is recommended that the BCLC staging system is used as the framework for HCC management in Australia |
B1 |
50 (94.0%) |
||||||||||||
|
12 |
The management choice for a patient with HCC should take into account the individual patient’s wishes and medical and psychosocial circumstances |
C1 |
50 (100%) |
||||||||||||
|
13 |
The management of HCC should be determined by a multidisciplinary team to optimise patient care |
B1 |
50 (100%) |
||||||||||||
|
14 |
Liver resection is a first‐line therapy option in suitable patients with HCC where there is preserved liver function, sufficient liver remnant, and absence of significant portal hypertension |
B1 |
48 (95.8%) |
||||||||||||
|
15 |
Liver transplantation should be considered for patients with HCC within transplant criteria who are not suitable for curative hepatic resection or ablative therapy |
A1 |
48 (100%) |
||||||||||||
|
16 |
UCSF criteria should inform patient selection for liver transplantation in patients with HCC |
B1 |
47 (100%) |
||||||||||||
|
17 |
Patients with HCC initially beyond transplant criteria may be considered for liver transplantation after successful downstaging to within standard transplant criteria |
C1 |
41 (97.6%) |
||||||||||||
|
18 |
Ablative therapy is recommended as a curative locoregional therapy in suitable patients with very early or early (BCLC stage 0 or A) HCC |
B1 |
39 (94.9%) |
||||||||||||
|
19 |
Patients with early stage disease (BCLC stage A and early stage B), who are not candidates for surgery or liver transplantation, should be treated with locoregional therapy |
A1 |
48 (100%) |
||||||||||||
|
20 |
In patients with BCLC‐B HCC, TACE is recommended as first‐line therapy |
B1 |
47 (97.9%) |
||||||||||||
|
21 |
SIRT may be considered in select patients with intermediate or locally advanced HCC |
C2 |
44 (88.6%) |
||||||||||||
|
22 |
Stereotactic external‐beam radiation therapy may be considered for local tumour control in suitable patients with HCC |
C2 |
42 (81.0%) |
||||||||||||
|
23 |
Patients with advanced HCC (BCLC‐C) or multifocal HCC that is not amenable to curative or locoregional therapy (BCLC‐B) should be offered systemic therapy |
A1 |
49 (93.9%) |
||||||||||||
|
24 |
Sorafenib or lenvatinib§ is recommended as initial systemic therapy in patients with advanced (BCLC‐C) or multifocal HCC that is not amenable to curative or locoregional therapy (BCLC‐B) and who have preserved liver function and good performance status |
A1 |
48 (100%) |
||||||||||||
|
25 |
The use of multikinase inhibitors as adjuvant therapy after hepatic resection or locoregional therapy is not recommended |
A1 |
44 (100%) |
||||||||||||
|
26 |
In patients with HCC, regular assessment for clinical and radiological response to first‐line therapy is recommended to monitor for disease progression |
A1 |
50 (100%) |
||||||||||||
|
27 |
In patients with HCC, sorafenib or lenvatinib should be discontinued when there is unequivocal clinical and/or radiological progression |
A1 |
45 (100%) |
||||||||||||
|
28 |
In patients with HCC, a second‐line systemic therapy is recommended in suitable patients who have radiological progression while being treated with multikinase inhibitors but preserved liver function and good performance status |
A1 |
41 (92.7%) |
||||||||||||
|
29 |
HCC treatment response should be assessed by multiphase CT or MRI using standardised criteria such as the mRECIST criteria |
B1 |
48 (81.3%) |
||||||||||||
|
30 |
Patients with incurable HCC should be introduced to supportive care services early in their management |
B1 |
50 (100%) |
||||||||||||
|
31 |
Patients with BCLC‐D HCC should be managed symptomatically in conjunction with supportive care services |
B1 |
50 (100%) |
||||||||||||
|
|
|||||||||||||||
|
BCLC = Barcelona Clinic Liver Cancer; CT = computed tomography; GRADE = Grading of Recommendations Assessment, Development and Evaluation; HBV = hepatitis B virus; HCV = hepatitis C virus; mRECIST = modified Response Evaluation Criteria in Solid Tumors; MRI = magnetic resonance imaging; SIRT = selective internal radiation therapy; TACE = transarterial chemoembolisation; UCSF = University of California San Francisco. * GRADE quality of evidence classification: A = high, B = moderate, C = low, D = very low; strength of recommendation: 1 = strong, 2 = weak. † Number of experts who participated in the final modified Delphi process vote for this recommendation. ‡ Percentage of expert advisors who either strongly agreed or agreed (based on five-point Likert scale, comprising: strongly disagree, disagree, neutral, agree and strongly agree). § Or combination of atezolizumab and bevacizumab (listed in the Pharmaceutical Benefits Scheme on 1 November 2020). Source: Hepatocellular Carcinoma Consensus Statement Working Group. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Melbourne: Gastroenterological Society of Australia, 2020. https://www.gesa.org.au/resources/hepatocellular-carcinoma-hcc-management-consensus/. [Corrections added on 12 May 2021 after first online publication: a footnote was added to recommendation 24 in Box 1.] |
|||||||||||||||
Box 2 – Populations that should undergo hepatocellular carcinoma surveillance
|
|
|||||||||||||||
|
People with cirrhosis (any aetiology) |
|||||||||||||||
|
People with chronic hepatitis B virus infection without cirrhosis in:
|
|||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 – Surveillance and diagnosis of hepatocellular carcinoma (HCC)

AFP = α-fetoprotein; BCLC = Barcelona Clinic Liver Cancer; CT = computed tomography; HBV = hepatitis B virus; MDT = multidisciplinary team; MRI = magnetic resonance imaging. All cirrhotic and non-cirrhotic patients at increased risk of HCC should be offered surveillance (if willing to receive treatment). The preferred surveillance method is liver ultrasound ± serum AFP every 6 months. Once a lesion is identified on ultrasound, depending on size, further evaluation is required with either multiphase CT or MRI. Depending on the imaging characteristics, lesions may be classified as: likely benign, intermediate, or definite HCC. Discussion in an MDT meeting is recommended in order to optimise patient care. Source: Hepatocellular Carcinoma Consensus Statement Working Group. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Melbourne: Gastroenterological Society of Australia, 2020. https://www.gesa.org.au/resources/hepatocellular-carcinoma-hcc-management-consensus/
Box 4 – Barcelona Clinic Liver Cancer (BCLC) staging system
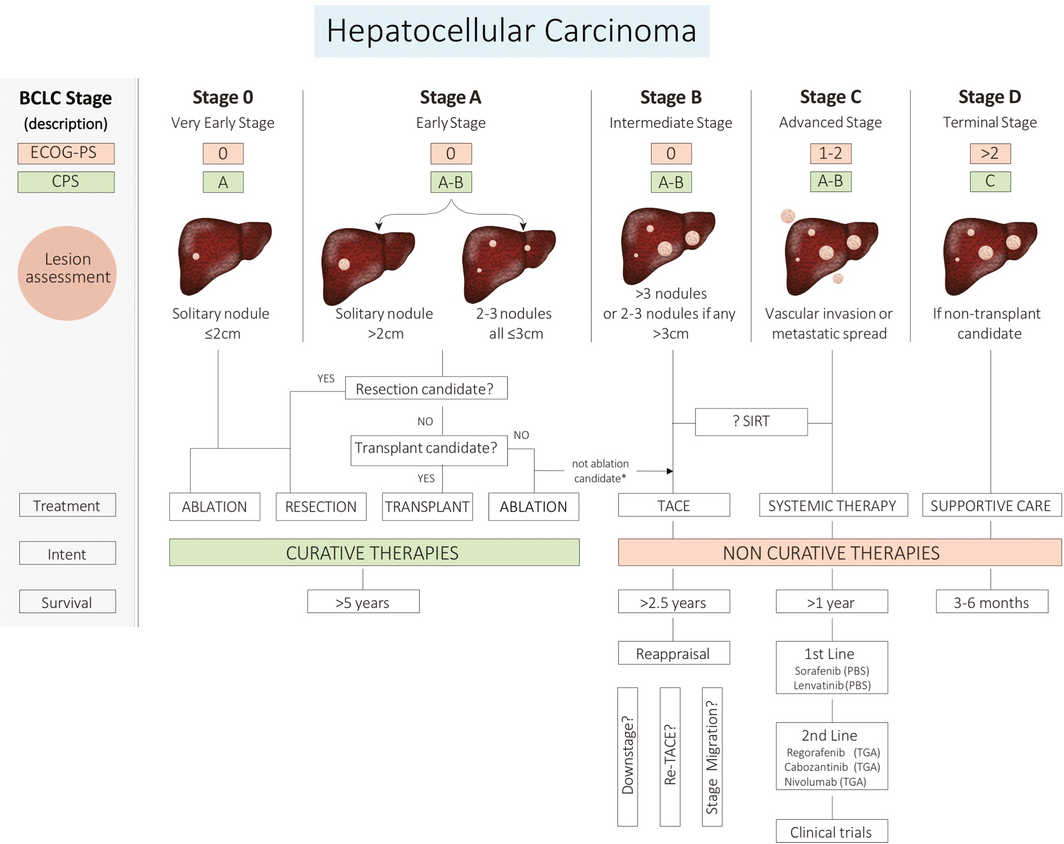
CPS = Child–Pugh Score; ECOG-PS = Eastern Cooperative Oncology Group Performance Status; PBS = Pharmaceutical Benefits Scheme; SIRT = selective internal radiation therapy; TACE = Transarterial chemoembolisation; TGA = Therapeutic Goods Administration. The BCLC staging system is the preferred system for classifying hepatocellular carcinoma; the stage is calculated from three clinical parameters: liver function, tumour characteristics, and functional status of the patient. Updated versions are available at http://www.bclc.cat/professional-area/management-of-hcc.html . Source: Hepatocellular Carcinoma Consensus Statement Working Group. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Melbourne: Gastroenterological Society of Australia, 2020. https://www.gesa.org.au/resources/hepatocellular-carcinoma-hcc-management-consensus/
Box 5 – Comparison of liver transplantation criteria for patients with hepatocellular carcinoma (in chronological order)
|
|
Milan31 |
UCSF32 |
Up‐to‐733 |
Metroticket 2.034 |
|||||||||||
|
|
|||||||||||||||
|
Criteria for liver transplantation |
Single lesion ≤ 50 mm |
Single lesion ≤ 65 mm |
Sum of the diameter of the largest tumour (in cm) and the total number of tumours ≤ 7 |
Predictive model based on HCC number, maximal size and Log10 of AFP* |
|||||||||||
|
Conditions |
No macrovascular invasion, no regional nodal disease, no distant metastases |
||||||||||||||
|
|
|||||||||||||||
|
AFP = α-fetoprotein; CT = computed tomography; HCC = hepatocellular carcinoma; UCSF = University of California San Francisco. * Online calculator available at: http://www.hcc-olt-metroticket.org. Source: Hepatocellular Carcinoma Consensus Statement Working Group. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Melbourne: Gastroenterological Society of Australia, 2020. https://www.gesa.org.au/resources/hepatocellular-carcinoma-hcc-management-consensus/ |
|||||||||||||||
Box 6 – Supportive care, hepatology and medical goals in hepatocellular carcinoma (HCC)
|
|
BCLC‐0/A |
BCLC‐B |
BCLC‐C |
BCLC‐D |
|||||||||||
|
|
|||||||||||||||
|
Description |
Very early/early stage |
Intermediate |
Advanced |
Terminal |
|||||||||||
|
Overall aim |
Curative intent |
Non‐curative, increased survival while considering trade‐offs of treatment side effects |
Non‐curative, increased life expectancy while considering trade‐offs of treatment side effects and symptom management |
Palliative care, symptom control |
|||||||||||
|
HCC extent |
Confined to liver and small volume of tumour |
Confined to liver and large or multinodular |
Vascular invasion or spread outside liver |
Defined by poor liver function or cancer‐related symptoms |
|||||||||||
|
Liver function |
Preserved (no portal hypertension in stage 0) |
Preserved |
Preserved |
Decompensated |
|||||||||||
|
Cancer‐related symptoms |
None (ECOG 0) |
None (ECOG 0) |
Mild (ECOG 1–2) |
Marked (ECOG 3–4) |
|||||||||||
|
Type of therapy offered |
Ablation/resection or transplantation |
TACE |
Systemic therapy |
Best supportive care |
|||||||||||
|
Expected median survival (with treatment) |
> 5 years |
> 2.5 years |
> 1 year |
< 3 months |
|||||||||||
|
Supportive care aspects |
|
|
|
|
|||||||||||
|
|
Discuss and consider advanced care directives |
||||||||||||||
|
Hepatology‐focused goals |
|
|
|||||||||||||
|
General medical issues |
|
|
|||||||||||||
|
|
|||||||||||||||
|
BCLC = Barcelona Clinic Liver Cancer; ECOG = Eastern Cooperative Oncology Group. Integration of BCLC staging system with supportive care needs, hepatological goals, and medical issues at an early stage. Source: Hepatocellular Carcinoma Consensus Statement Working Group. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Melbourne: Gastroenterological Society of Australia, 2020. https://www.gesa.org.au/resources/hepatocellular-carcinoma-hcc-management-consensus/ |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- John S Lubel1,2
- Stuart K Roberts1
- Simone I Strasser3,4
- Alexander J Thompson5
- Jennifer Philip6,7
- Mark Goodwin8
- Stephen Clarke9
- Darrell HG Crawford10
- Miriam T Levy11
- Nick Shackel12
- 1 Alfred Health, Melbourne, VIC
- 2 Monash University, Melbourne, VIC
- 3 AW Morrow Gastroenterology and Liver Centre, Royal Prince Alfred Hospital, Sydney, NSW
- 4 University of Sydney, Sydney, NSW
- 5 St Vincent’s Hospital, Melbourne, VIC
- 6 University of Melbourne, Melbourne, VIC
- 7 Royal Melbourne Hospital, Melbourne, VIC
- 8 Austin Hospital, Melbourne, VIC
- 9 Royal North Shore Hospital, Sydney, NSW
- 10 Greenslopes Private Hospital, University of Queensland, Brisbane, QLD
- 11 Liverpool Hospital Sydney, Sydney, NSW
- 12 University of New South Wales, Sydney, NSW
We acknowledge the sponsors for this project, who provided unrestricted financial support to the Gastroenterological Society of Australia (GESA) for the logistics of this project. We confirm sponsors have not had or sought involvement in the selection of the project, design, conduct, analysis or outcomes, or articulation of the results. Sponsors have not been involved with the project committee directly, although they may have been involved through normal operations with project participants (see Competing interests declaration).
The sponsors were: Bayer, Medtronic Australasia, Sirtex Medical, Eisia, Novartis and Gilead Sciences.
Infrastructural and administration support was provided by GESA with absolutely no involvement with writing, editing or manuscript generation.
We also acknowledge the following contributors to the development of the document, including involvement with the modified Delphi process: Leon Adams, Golo Ahlenstiel, Peter Angus, Sally Bell, James Burnes, Sarat Chander, Robert Cheng, Asif Chinnaratha, Maria Cigolini, Paul Clark, Olivia Cullen, Anouk Dev, Greg Dore, Michael Fink, Jacob George, Paul Gow, Ingrid Hickman, Thai Hong, Jessica Howell, Ashu Jhamb, David Iser, Will Kemp, Lara Lipton, Gerry MacQuillan, Geoff McCaughan, Kate Muller, Barbara Moore, Michael Ng, Amanda Nicoll, John Olynyk, David Pryor, Kate Reed‐Cox, Chris Rogan, Marno Ryan, Glen Schlaphoff, James Seow, William Sievert, Manfred Spanger, Sally Spruce, Katherine Stuart, Tom Sutherland, Caroline Tallis, Niall Tebbutt, Michael Wallace, Martin Weltman, Allan Wigg and Amany Zekry.
Nick Shackel has been on advisory boards and a speaker for Roche, BMS, Gilead, Bayer, Astelis and Novartis. Stuart Roberts has been on advisory boards for Gilead, AbbieVie, BMS (HCC) and MSD. Alex Thompson has received payment and research funding from Gilead and BMS. Simone Strasser has received honoraria for advisory boards and speaker fees from Bayer, Sirtex, Gilead, AbbVie, Bristol Myers Squibb, MSD, Norgine, Astellas, Novartis, Eisai, Ipsen and Pfizer. Mark Goodwin has been paid as a consultant for Bayer and Sirrex Medical (Proctor). Stephen Clarke has been on the Bayer board. Darrell Crawford serves on an Eisai Advisory Board.
- 1. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926.
- 2. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol 2010; 63: 1308–1311.
- 3. Gustafson DH, Shukla RK, Delbecq A, et al. A comparative study of differences in subjective likelihood estimates made by individuals, interacting groups, Delphi groups, and nominal groups. Organ Behav Hum Perform 1973; 9: 280–291.
- 4. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941–1953.
- 5. Wallace MC, Preen DB, Short MW, et al. Hepatocellular carcinoma in Australia 1982–2014: increasing incidence and improving survival. Liver Int 2019; 39: 522–530.
- 6. MacLachlan JH, Cowie BC. Liver cancer is the fastest increasing cause of cancer death in Australians. Med J Aust 2012; 197: 492–493. https://www.mja.com.au/journal/2012/197/9/liver-cancer-fastest-increasing-cause-cancer-death-australians
- 7. Australian Institute of Health and Welfare. Cancer in Australia 2019 [Cat. No. CAN 123]. Canberra: AIHW; 2019. https://www.aihw.gov.au/reports/cancer/cancer-in-australia-2019/contents/table-of-contents (viewed Nov 2020).
- 8. Hong TP, Gow P, Fink M, et al. Novel population‐based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology 2016; 63: 1205–1212.
- 9. Howell J, Pedrana A, Cowie BC, et al. Aiming for the elimination of viral hepatitis in Australia, New Zealand, and the Pacific Islands and Territories: where are we now and barriers to meeting World Health Organization targets by 2030. J Gastroenterol Hepatol 2019; 34: 40–48.
- 10. Graham S, Guy RJ, Cowie B, et al. Chronic hepatitis B prevalence among Aboriginal and Torres Strait Islander Australians since universal vaccination: a systematic review and meta‐analysis. BMC Infect Dis 2013; 13: 403.
- 11. Parker C, Tong SY, Dempsey K, et al. Hepatocellular carcinoma in Australia’s Northern Territory: high incidence and poor outcome. Med J Aust 2014; 201: 470–474. https://www.mja.com.au/journal/2014/201/8/hepatocellular-carcinoma-australias-northern-territory-high-incidence-and-poor
- 12. Sarasin FP, Giostra E, Hadengue A. Cost‐effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child‐Pugh class A cirrhosis. Am J Med 1996; 101: 422–434.
- 13. El‐Serag HB, Kanwal F, Richardson P, et al. Risk of hepatocellular carcinoma after sustained virological response in veterans with hepatitis C virus infection. Hepatology 2016; 64: 130–137.
- 14. Hong TP, Gow PJ, Fink M, et al. Surveillance improves survival of patients with hepatocellular carcinoma: a prospective population‐based study. Med J Aust 2018; 209: 348–354. https://www.mja.com.au/journal/2018/209/8/surveillance-improves-survival-patients-hepatocellular-carcinoma-prospective#:~:text=Median%20overall%20survival%20time%20was,CI%2C%200.19%E2%80%930.58
- 15. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona‐2000 EASL conference. J Hepatol 2001; 35: 421–430.
- 16. Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta‐analysis of cohort studies. Br J Cancer 2007; 97: 1005–1008.
- 17. Saunders D, Seidel D, Allison M, et al. Systematic review: the association between obesity and hepatocellular carcinoma — epidemiological evidence. Aliment Pharmacol Ther 2010; 31: 1051–1063.
- 18. El‐Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case–control study among United States Veterans. Am J Gastroenterol 2001; 96: 2462–2467.
- 19. Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta‐analysis of cohort studies. Int J Cancer 2012; 130: 1639–1648.
- 20. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non‐alcoholic fatty liver disease. Gastroenterology 2018; 155: 1828–1837.e2.
- 21. Purysko AS, Remer EM, Coppa CP, et al. LI‐RADS: a case‐based review of the new categorization of liver findings in patients with end‐stage liver disease. Radiographics 2012; 32: 1977–1995.
- 22. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022.
- 23. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 2014; 273: 30–50.
- 24. Roberts LR, Sirlin CB, Zaiem F, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta‐analysis. Hepatology 2018; 67: 401–421.
- 25. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236.
- 26. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018; 391: 1301–1314.
- 27. Taylor C, Munro AJ, Glynne‐Jones R, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ 2010; 340: c951.
- 28. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev 2016; 42: 56–72.
- 29. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014; 21: 1287–1295.
- 30. Earl TM, Chapman WC. Hepatocellular carcinoma: resection versus transplantation. Semin Liver Dis 2013; 33: 282–92.
- 31. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699.
- 32. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33: 1394–1403.
- 33. Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009; 10: 35–43.
- 34. Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018; 154: 128–139.
- 35. Roberts SK, Gazzola A, Lubel J, et al. Treatment choice for early‐stage hepatocellular carcinoma in real‐world practice: impact of treatment stage migration to transarterial chemoembolization and treatment response on survival. Scand J Gastroenterol 2018; 53: 1368–1375.
- 36. Breen DJ, Lencioni R. Image‐guided ablation of primary liver and renal tumours. Nat Rev Clin Oncol 2015; 12: 175–186.
- 37. Boutros C, Somasundar P, Garrean S, et al. Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol 2010; 19: e22–e32.
- 38. Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009; 49: 453–459.
- 39. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67: 358–380.
- 40. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37: 429–442.
- 41. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo‐controlled, double‐blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017; 2: 565–575.
- 42. Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia‐Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018; 36: 1913–1921.
- 43. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium‐90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open‐label randomised controlled phase 3 trial. Lancet Oncol 2017; 18: 1624–1636.
- 44. Ricke J, Klumpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019; 71: 1164–1174.
- 45. Dobrzycka M, Spychalski P, Rostkowska O, et al. Stereotactic body radiation therapy for early‐stage hepatocellular carcinoma ‐ a systematic review on outcome. Acta Oncol 2019; 58: 1706–1713.
- 46. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol 2018; 4: 661–669.
- 47. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390.
- 48. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet 2018; 391: 1163–1173.
- 49. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905.
- 50. Cabibbo G, Enea M, Attanasio M, et al. A meta‐analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010; 51: 1274–1283.
- 51. Bosilkovska M, Walder B, Besson M, et al. Analgesics in patients with hepatic impairment: pharmacology and clinical implications. Drugs 2012; 72: 1645–1669.





Abstract
Introduction: Hepatocellular carcinoma (HCC) is a leading cause of cancer deaths both globally and in Australia. Surveillance for HCC in at‐risk populations allows diagnosis at an early stage, when potentially curable. However, most Australians diagnosed with HCC die of the cancer or of liver disease. In the changing landscape of HCC management, unique challenges may lead to clinical practice variation. As a result, there is a need to identify best practice management of HCC in an Australian context. This consensus statement has been developed for health professionals involved in the care of adult patients with HCC in Australia. It is applicable to specialists, general medical practitioners, nurses, health coordinators and hospital administrators.
Methods and recommendations: This statement has been developed by specialists in hepatology, radiology, surgery, oncology, palliative care, and primary care, including medical practitioners and nurses. The statement addresses four main areas relevant to HCC management: epidemiology and incidence, diagnosis, treatment, and patient management.
A modified Delphi process was used to reach consensus on 31 recommendations. Principal recommendations include the adoption of surveillance strategies, use of multidisciplinary meetings, diagnosis, treatment options and patient management.
Changes in management as a result of this statement: This consensus statement will simplify HCC patient management and reduce clinical variation. Ultimately, this should result in better outcomes for patients with HCC.