The known: In February 2018, the Australian Therapeutic Goods Administration rescheduled codeine‐containing products as prescription only medicines because of misuse and dependence problems.
The new: After the rescheduling of codeine‐containing analgesics, over‐the‐counter sales to pharmacies of paracetamol, paracetamol combinations, and ibuprofen products increased. In contrast, sales of prescription analgesics did not rise. Sales of over‐the‐counter cold/flu products containing the opioid derivative dextromethorphan increased slightly, but not those of cough products containing dextromethorphan, dihydrocodeine, or pholcodine.
The implications: Rescheduling codeine was followed by market substitution by over‐the‐counter analgesics with lower risks of dependence, primarily single ingredient paracetamol and ibuprofen.
Codeine is the most used opioid in Australia; in 2013, nearly 28 million packs of codeine‐containing products were sold, including 15 million over‐the‐counter (OTC) products available without prescription.1 As with other opioids, prolonged codeine use entails a risk of tolerance and dependence. Between 2000 and 2009, codeine‐related mortality in Australia increased from 3.5 to 8.7 deaths per million population;2 the number of people seeking treatment for codeine dependence has also increased substantially.3,4
From 2010 to early 2018, low strength codeine products (up to 15 mg codeine/unit) could be bought from pharmacies in Australia without prescription, although some products required that the purchaser consult a pharmacist. Combinations of codeine with paracetamol or non‐steroidal anti‐inflammatory drugs (NSAIDs) were available over the counter, although the analgesic benefit of low strength codeine combination products at recommended doses is only slightly greater than that of paracetamol or NSAIDs alone.5,6 When codeine combination products are taken at higher than recommended doses, the large amounts of paracetamol or NSAIDs ingested may increase the risk of adverse events associated with these drugs, such as gastrointestinal bleeding and liver failure.7 Further, individual responses to codeine are highly variable because of genetic variability in metabolic pathways;8 some people obtain inadequate pain relief, while others (ultrarapid metabolisers) are at increased risk of adverse events.9
To curb rising levels of codeine misuse and related harm,10 the Australian Therapeutic Goods Administration (TGA) rescheduled all combination codeine products as prescription only medicines from February 2018.11 While the Australian Medical Association12 and the Royal Australian College of General Practitioners13 supported the change, pharmacy groups were concerned that it would impede access to codeine for people with legitimate pain relief needs.14 The number of codeine‐related calls to the New South Wales Poisons Information Centre substantially declined after rescheduling, and sales of low strength codeine products declined by 87%, without high strength codeine sales rising.15
It is not known whether the rescheduling of codeine as a prescription only medicine in February 2018 has affected the sales of OTC products that people might substitute for codeine. The objective of our investigation was therefore to quantify changes in pharmacy purchases of OTC and prescription analgesics, cold and flu products, and cough suppressants after the rescheduling of codeine.
Methods
Data source
To estimate total sales to community pharmacies of OTC and prescription analgesics, cold and flu products, and cough suppressants, with and without codeine, we analysed Australian pharmacy sales data for the period March 2015 – March 2019, provided by IQVIA (iqvia.com), a health information company that maintains a database of pharmaceutical manufacturer sales. The IQVIA data capture about 96% of all OTC and prescription pharmaceutical sales to pharmacies in Australia. We restricted our study to sales to community pharmacies, excluding sales to hospitals. Data for sales to community vendors other than pharmacies (eg, packs of 20 or fewer paracetamol tablets and 24 or fewer tablets of ibuprofen sold to supermarkets) were not available.
Rescheduling of codeine
Codeine products are available for treating pain, alone and in combination with paracetamol, ibuprofen, doxylamine/paracetamol, or aspirin; for treating cold and flu symptoms, in various combinations with an analgesic, antihistamine, decongestant, or cough suppressant; and as a linctus (syrup) for cough suppression. Before 1 February 2018, tablet and capsule formulations containing no more than 15 mg codeine phosphate could be purchased without prescription from pharmacies. Packs of tablets or capsules containing no more than 15 mg codeine phosphate and no more than 500 mg in total were classified as Schedule 3 (pharmacist only) medicines and required consultation with a pharmacist, while packs of cold and flu products containing no more than 10 mg codeine per tablet/capsule and no more than 360 mg in total were classified as Schedule 2 (pharmacy only) or Schedule 3 medicines. Combination analgesic products including 30 mg codeine are Schedule 4 (prescription only) medicines, while single ingredient codeine tablets and codeine linctus are Schedule 8 (controlled drug) medicines, with additional restrictions on their prescribing and dispensing (Supporting Information, table 1). On 20 December 2016, the TGA announced that OTC codeine products would be rescheduled as Schedule 4 medicines from 1 February 2018; OTC products containing opioid derivatives (pholcodine, dextromethorphan, dihydrocodeine) were not rescheduled.
Other medicines of interest
We included all OTC and prescription analgesics, analgesic‐containing products for the treatment of cold and flu symptoms, and cough suppressants. We defined OTC medicines as including those classified as Schedule 2 or 3 medicines and unscheduled medicines available for purchase without prescription. Veterans, people in palliative care, and Aboriginal and Torres Strait Islander Australians can receive selected OTC medicines through the Pharmaceutical Benefits Scheme (PBS) or Repatriation Schedule of Pharmaceutical Benefits. For the general population, some formulations of paracetamol could also be obtained through the PBS prior to 2016; however, paracetamol was among the OTC products delisted by the PBS in January 2016.16 For our analysis of analgesics, we only included tablet and capsule formulations (excluding, for example, transdermal patches, oral solutions, injections). Most non‐tablet/capsule OTC analgesic products were oral solutions for children, while most non‐tablet/capsule prescription analgesic products were transdermal patches for severe chronic pain; neither type is likely to be substituted for OTC codeine products. Data on topical anti‐inflammatory agents (eg, diclofenac gel) were not available. For our analysis of cold and flu and cough products, we included tablets, capsules, liquids, and syrups, but excluded lozenges and powders.
We included the following non‐codeine‐containing OTC analgesics: paracetamol, alone or in combination with ibuprofen, diphenhydramine, caffeine, or metoclopramide; ibuprofen; aspirin; diclofenac; naproxen; and mefenamic acid. We also explored changes in sales of prescription analgesics: NSAIDs, antimigraine medicines (but not preventives), gabapentinoids (pregabalin, gabapentin) for neuropathic pain, tramadol, and strong opioids (eg, oxycodone, morphine, tapentadol). Apart from codeine, tramadol is the only Schedule 4 opioid and is a logical substitute for codeine; we analysed it separately from the Schedule 8 opioids. Data on opioids used for treating opioid dependence (eg, methadone, buprenorphine, buprenorphine/naloxone) were not available (Supporting Information, table 2).
We also included all analgesic‐containing OTC products used to treat cold and flu symptoms, including combinations of analgesics, antihistamines, decongestants, cough suppressants, and expectorants (Supporting Information, table 3). Data on cold and flu products not including an analgesic (eg, pure decongestants) were not available. We also included OTC cough suppressants, which are primarily supplied as liquids or syrups and typically contain dextromethorphan or pholcodine, either alone or in combination with a mucolytic, antihistamine, expectorant, or decongestant. Dihydrocodeine (Schedule 3) is sold over the counter as a single ingredient cough suppressant. Misuse of the opioid derivatives dextromethorphan and dihydrocodeine has been reported.7,17 There were no codeine‐containing OTC cough suppressants, but single ingredient codeine linctus is available on prescription.
Statistical analysis
To quantify changes in analgesic sales, we summed the total sales to pharmacies per month, by OTC or prescription status and specific medicine, and calculated sales per 10 000 population, based on Australian Bureau of Statistics mid‐year populations.18 We measured codeine sales in numbers of packs, tablets/capsules, and kilograms per 10 000 population. For comparing different medicines, we chose tablets/capsules as the primary unit of analysis because pack sizes differ (eg, codeine/paracetamol is primarily supplied in packs of 20 or 30, while single ingredient paracetamol is chiefly supplied by pharmacies in packs of 96 or 100). For our analysis of all OTC analgesics, we also summed the total number of packs per month. For cold and flu and cough products, we summed the total number of packs, as non‐tablet/capsule formulations were common. We compared sales of analgesic, cold and flu, and cough products in the 12 months prior to the rescheduling announcement, and 12 months after the rescheduling.
We used interrupted time series analysis to quantify changes in monthly sales to pharmacies after rescheduling in February 2018. To account for autocorrelation, we used an autoregressive model; that is, a model in which the outcome values are regressed on previous values in the series. To control for seasonality, we used two different approaches: for analgesic sales, we included dummy variables for the months of the year; for cold and flu products and cough suppressants, with undulating sales patterns (higher in winter, lower in summer), we included Fourier terms. As sales patterns changed in anticipation of rescheduling,15 we excluded the period between the TGA announcement and rescheduling (January 2017 – January 2018) from the time series analysis.19 We compared sales after rescheduling with expected sales had the trend prior to the announcement continued, and estimated the pre‐announcement (baseline) monthly gradient (slope) in sales data, the level shift in sales numbers after rescheduling (ie, difference between observed and predicted values based on pre‐announcement trend), and the change in slope after rescheduling. A level shift indicates an immediate and sustained change in sales, while a change in slope reflects a gradual change (Supporting Information, supplementary methods). All analyses were performed in SAS 9.4 and R 3.3.1.
Ethics approval
Ethics approval was not required for our analysis of population‐level (non‐identifiable) data.
Results
Sales of analgesic‐containing pharmaceuticals to community pharmacies accounted for 92% of sales volume in this category (511 157 947 units; IQVIA data), excluding sales to hospitals. Of sales to community pharmacies, 86% of OTC analgesics (227 153 335 units) and 91% of prescription analgesics (179 032 423 units) were in the form of tablets or capsules. During 1 January – 31 December 2016, 24 285 packs and 1 396 650 tablets/capsules of OTC analgesics were sold per 10 000 population, 3630 packs of OTC cold/flu medicines per 10 000 population, and 1134 packs of OTC cough suppressant sales per 10 000 population. Codeine‐containing products comprised 31.2% of all OTC analgesic pack sales and 17.8% of analgesic tablet/capsule sales, and 69.7% of OTC cold/flu product pack sales (Box 1, Box 2).
Prior to the announcement, OTC analgesic sales were declining by 1457 tablets per 10 000 population per month (95% confidence interval [CI], 1211–1703 per 10 000 population per month). A level shift in monthly sales was evident during the first 14 months after rescheduling: 37 856 more tablets per 10 000 population (95% CI, 26 143–49 569 per 10 000 population) were sold than predicted had the pre‐announcement movement in sales continued. Monthly pack sales also increased after rescheduling, by 258 packs per 10 000 population (95% CI, 151–365 per 10 000 population). No changes in slope were associated with rescheduling (Box 3, Box 4).
Sales of codeine‐containing analgesic products
During 2016, 7586 packs, 248 127 tablets/capsules, and 3050 kilograms per 10 000 population of OTC codeine‐containing analgesic products were sold to pharmacies, as were 5302 packs, 106 621 tablets/capsules, and 3199 kilograms per 10 000 population of prescription codeine‐containing analgesics. The most frequently sold codeine product was codeine/paracetamol (51% of OTC, 95% of prescription codeine‐containing analgesic tablets/capsules) (Box 1). Following the rescheduling announcement, OTC codeine sales increased, with sales in August 2017 nearly twice the predicted values (Box 5).
After rescheduling, small shifts in the levels of monthly sales of prescription codeine products (tablets/capsules: increase of 2247 tablets per 10 000 population; 95% CI, 1231–3264 per 10 000 population; packs: increase of 84 packs; 95% CI, 34–134 per 10 000 population) were noted, but there was no significant change in the number of kilograms sold (increase of 23 kg per 10 000 population; 95% CI, –7 to 53 kg per 10 000 population). No changes in slope were associated with rescheduling (Box 4). The increase in volume of prescription analgesic sales was lower than the volume of eliminated OTC codeine sales (Box 1).
Sales of analgesic products not containing codeine
During 2016, 16 699 packs and 1 147 523 tablets per 10 000 population of OTC analgesic products not including codeine were sold to pharmacies (Box 1). Single ingredient paracetamol was the most frequently sold analgesic during 2016. (44.9% of packs, 67.0% of tablets/capsules of OTC analgesic products; Supporting Information, table 4). In the year after rescheduling, the market share of paracetamol increased to 60.1% of packs and 79.2% of tablets/capsule sales; ibuprofen (18.1% of packs, 12.9% of tablets/capsules) and paracetamol/ibuprofen (8.3% of packs, 2.2% of tablets/capsules) were the next most frequently sold products. The greatest level increase in monthly sales after rescheduling was for single ingredient paracetamol (increase of 41 415 tablets per 10 000 population; 95% CI, 31 374–51 456 per 10 000 population), with no change in slope (Box 4, Box 6). Level shifts were also evident for ibuprofen, aspirin, and paracetamol combinations (most frequently sold: paracetamol/ibuprofen), but not for any prescription analgesics, including tramadol and strong opioids; there was a slight increase in slope but no level shift for diclofenac sales (Box 4; Supporting Information, figure 1).
Cold and flu products and cough suppressants
During 2016, 3630 OTC cold and flu product packs were sold per 10 000 population, of which 2531 packs (69.7%) were codeine‐containing products (Box 2). During the year after rescheduling, sales of OTC cold and flu products not including codeine increased by 146.4 packs per 10 000 population (95% CI, 128.0–164.8 per 10 000 population; Supporting Information, table 5). While dextromethorphan‐containing cold and flu product sales increased (by 22.6 packs per 10 000 population; 95% CI, 15.2–30.1 per 10 000 population), the increase for cold and flu products not including dextromethorphan was greater (123.5 packs per 10 000 population; 95% CI, 112.9–134.0 per 10 000 population; Supporting Information, table 5 and figure 2). Sales to pharmacies of OTC cough suppressants, including those containing opioid derivatives (dextromethorphan, dihydrocodeine, pholcodine), did not change (Supporting Information, table 6 and figure 3).
Discussion
In the first year after codeine‐containing products were rescheduled as prescription only medicines, sales to pharmacies of OTC non‐opioid analgesics increased. In contrast, prescription codeine sales increased only slightly. Sales of OTC cold and flu products including the opioid derivative dextromethorphan increased slightly, while sales of OTC cough suppressants containing opioid derivatives did not increase at all. The largest increases in sales were for paracetamol, alone or in combination with ibuprofen. The analgesic effect of the combination of paracetamol and ibuprofen is comparable with that of codeine combined with paracetamol or ibuprofen.5,6 The large relative increase in sales of combination paracetamol/ibuprofen products may be linked with increased marketing after the rescheduling of codeine, as well as with the convenience of their OTC availability in pharmacies. Growing awareness of the harms of opioid overprescribing may partly explain why sales of prescription products including opioids other than codeine did not increase.
Paracetamol is typically sold in larger pack sizes (96 or 100 tablets in pharmacies) than codeine, which explains the large increase in overall analgesic tablet sales after rescheduling. While paracetamol is not associated with dependence, excessive use can lead to serious adverse events, including liver failure. Sales of ibuprofen and ibuprofen‐containing products also increased. NSAIDs can cause gastrointestinal problems or renal impairment, especially if used in combination with other commonly prescribed medicines, particularly diuretic and antihypertensive agents that affect the renin–angiotensin system.20,21 Prior to the rescheduling of codeine, rates of paracetamol overdose‐related hospital admissions and liver injury had been increasing in Australia,22 and paracetamol‐ and NSAID‐related adverse events should be monitored.
Contrary to expectations, the increase in sales to pharmacies of prescribed codeine‐containing products after rescheduling was modest. We have previously reported that sales of prescription only, low strength preparations (maximum 15 mg codeine) increased but not those of higher strength preparations,15 suggesting that codeine use had partly been driven by the conveniently easy access of low strength preparations. Further, concern had been expressed that people might switch to other OTC products with potential for misuse, but sales of OTC products that include opioid derivatives (dextromethorphan, dihydrocodeine) did not markedly rise after the rescheduling of codeine. We have also reported that calls to Poisons Information Centres regarding these products had not increased.15
The large increase in OTC codeine sales to pharmacies between the rescheduling announcement in December 2016 and its implementation in February 2018 is consistent with reports of stockpiling,23 which may have been influenced by consumer fears about increased costs and difficulties in managing pain after the rescheduling.24 However, codeine is not regarded as appropriate for treating chronic pain; an anticipated outcome of rescheduling was that increased interactions with health practitioners would improve pain management, as well as reduce the risks of drug dependence.11 It is unclear whether rescheduling alone is sufficient to achieve these aims; complementary strategies, including greater funding for chronic pain management and dependence treatment programs, will be critical to improving health outcomes.
Limitations
While data on pharmaceutical sales to pharmacies are the best available for assessing OTC product sales in Australia, they are nonetheless limited in scope. These data do not measure sales to consumers by pharmacies, but are likely to reflect the levels of such sales. We could not assess harms associated with non‐medical use of codeine and other medicines, and we do not know whether people who were codeine‐dependent have received appropriate care. Further, without person‐level data we could not examine changes in groups at higher risk of codeine‐related harm, nor could we describe drug switching patterns, discontinuation, or the taking of multiple medicines by patients. Moreover, our analysis did not include data on smaller packs of paracetamol and ibuprofen products sold, for example, in supermarkets. The proportion of analgesic sales to vendors other than pharmacies is unknown, and it is likely that we have underestimated the increase in sales of OTC products.
Conclusions
Sales to pharmacies of OTC products not containing codeine, including paracetamol, ibuprofen, and paracetamol combinations, increased after the rescheduling of codeine‐containing products as prescription only medicines. Importantly, prescription codeine sales only increased slightly, as did sales of OTC products including opioid derivatives with potential for misuse. Changes in population rates of adverse events associated with paracetamol and ibuprofen should be monitored.
Box 1 – Sales to pharmacies of over‐the‐counter and prescription analgesics before and after rescheduling of codeine as a prescription only medicine
|
Analgesic products |
Sales, per 10 000 population |
||||||||||||||
|
12 months preceding rescheduling announcement* |
12 months after rescheduling* |
||||||||||||||
|
Packs |
Tablets and capsules |
Kilograms |
Packs |
Tablets and capsules |
Kilograms |
||||||||||
|
|
|||||||||||||||
|
Over‐the‐counter medicines |
24 285 |
1 396 650 |
NC |
19 576 |
1 291 277 |
NC |
|||||||||
|
Non‐codeine analgesics |
16 699 (68.8%) |
1 147 523 (82.2%) |
NC |
19 576 (100%) |
1 291 277 (100%) |
NC |
|||||||||
|
Codeine‐containing analgesics |
7586 (31.2%) |
248 127 (17.8%) |
3050 |
0 |
0 |
0 |
|||||||||
|
Codeine/paracetamol |
3601 (14.8%) |
125 573 (9.0%) |
1603 |
0 |
0 |
0 |
|||||||||
|
Codeine/ibuprofen |
2497 (10.3%) |
73 541 (5.3%) |
486 |
0 |
0 |
0 |
|||||||||
|
Codeine/doxylamine/paracetamol |
1401 (5.8%) |
47 551 (3.4%) |
941 |
0 |
0 |
0 |
|||||||||
|
Codeine/aspirin |
87 (0.4%) |
2462 (0.2%) |
20 |
0 |
0 |
0 |
|||||||||
|
Prescription medicines |
5302 |
106 621 |
3199 |
6949 |
147 821 |
3575 |
|||||||||
|
Codeine/paracetamol |
5060 (95.4%) |
101 412 (95.1%) |
3042 (95.1%) |
6007 (86.4%) |
124 867 (84.5%) |
3219 (90.0%) |
|||||||||
|
Codeine/doxylamine/paracetamol |
161 (3.0%) |
3225 (3.0%) |
97 (3.0%) |
572 (8.2%) |
12 434 (8.4%) |
201 (5.6%) |
|||||||||
|
Codeine |
81 (1.5%) |
1984 (1.9%) |
60 (1.9%) |
90 (1.3%) |
2072 (1.4%) |
58 (1.6%) |
|||||||||
|
Codeine/ibuprofen |
0 |
0 |
0 |
261 (3.8%) |
7701 (5.2%) |
91 (2.5%) |
|||||||||
|
Codeine/aspirin |
0 |
0 |
0 |
19 (0.3%) |
747 (0.5%) |
6 (0.2%) |
|||||||||
|
|
|||||||||||||||
|
NC = not calculated, because not comparable for different over‐the‐counter analgesics. * Rescheduling announced: 20 December 2016; rescheduling effective: 1 February 2018. |
|||||||||||||||
Box 2 – Sales to pharmacies of cold and flu products and cough suppressants before and after after rescheduling of codeine as a prescription only medicine
|
|
Sales, packs per 10 000 population |
||||||||||||||
|
12 months preceding rescheduling announcement |
12 months after rescheduling |
||||||||||||||
|
|
|||||||||||||||
|
Over‐the‐counter cold/flu products |
|
|
|||||||||||||
|
All |
3630 |
2768 |
|||||||||||||
|
Codeine‐containing |
2531 (69.7%) |
0 |
|||||||||||||
|
Dextromethorphan‐containing* |
390 (10.8%) |
546 (19.7%) |
|||||||||||||
|
Other |
709 (19.5%) |
2222 (80.3%) |
|||||||||||||
|
Over‐the‐counter cough suppressants |
|
|
|||||||||||||
|
All |
1134 |
1134 |
|||||||||||||
|
Pholcodine‐containing |
597 (52.7%) |
599 (52.8%) |
|||||||||||||
|
Dextromethorphan‐containing |
299 (26.4%) |
266 (23.5%) |
|||||||||||||
|
Dihydrocodeine |
238 (20.9%) |
267 (23.5%) |
|||||||||||||
|
Other |
1 (0.1%) |
3 (0.2%) |
|||||||||||||
|
Prescription cough suppressants |
|
|
|||||||||||||
|
Codeine linctus |
39 |
36 |
|||||||||||||
|
|
|||||||||||||||
|
* Products containing both codeine and dextromethorphan are included under “codeine‐containing”. |
|||||||||||||||
Box 3 – Monthly sales to pharmacies of all over‐the‐counter analgesic products, 2015–2019, by tablets/capsules and packs
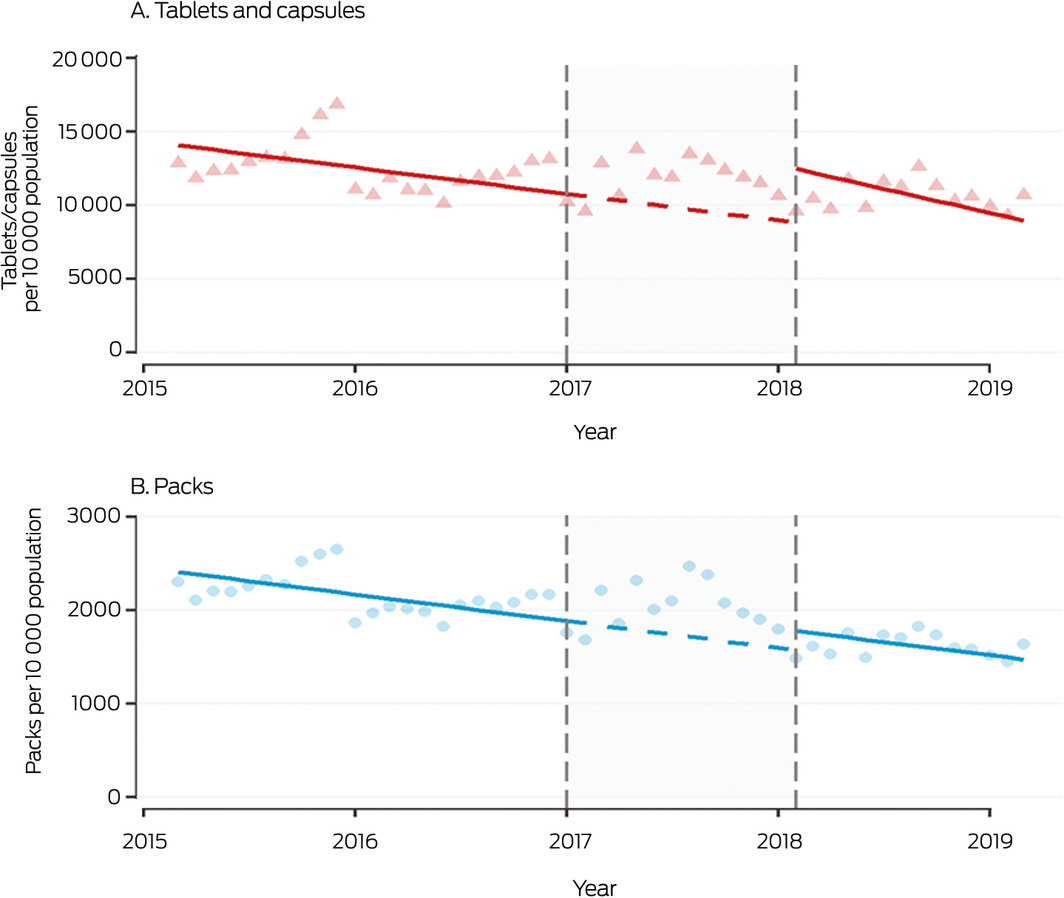
Shaded area: period between announcement (20 December 2016) and implementation (1 February 2018) of rescheduling of codeine‐containing products. Symbols: monthly values; solid line: fitted straight line trend; dashed line: continuation of pre‐announcement trend into period between announcement and implementation of rescheduling. The high rate of analgesic sales at the end of 2015 may reflect stockpiling of Pharmaceutical Benefits Scheme‐subsidised paracetamol before its delisting in January 2016 for the general population.
Box 4 – Monthly sales to pharmacies of over‐the‐counter and prescription analgesic products (tablets and capsules), 2015–2019: autoregressive error model, adjusted for seasonality
|
|
Slope (95% CI), tablets/capsules per 10 000 population per month |
Level shift* after rescheduling (95% CI), tablets/capsules per 10 000 population |
|||||||||||||
|
Before announcement |
Change after rescheduling (95% CI) |
||||||||||||||
|
|
|||||||||||||||
|
Prescription codeine products |
|
|
|
||||||||||||
|
Tablets/capsules |
12 (–20 to 44) |
–15 (–87 to 57) |
2247 (1231 to 3264) |
||||||||||||
|
Packs |
1 (–1 to 2) |
–1 (–5 to 2) |
84 (34 to 134) |
||||||||||||
|
Kilograms |
0 (–1 to 1) |
–1 (–3 to 1) |
23 (–7 to 53) |
||||||||||||
|
Over‐the‐counter analgesics |
|
|
|
||||||||||||
|
All |
–1457 (–1703 to –1211) |
–1229 (–2496 to 37) |
37 856 (26 143 to 49 569) |
||||||||||||
|
Paracetamol |
–1209 (–1485 to –933) |
–287 (–1065 to 491) |
41 415 (31 374 to 51 456) |
||||||||||||
|
Ibuprofen |
13 (–3 to 29) |
55 (10 to 100) |
1427 (900 to 1954) |
||||||||||||
|
Paracetamol/ibuprofen |
7 (4 to 9) |
–37 (–41 to –32) |
1618 (1567 to 1669) |
||||||||||||
|
Other paracetamol combinations† |
–1 (–5 to 3) |
18 (9 to 26) |
233 (112 to 353) |
||||||||||||
|
Diclofenac |
8 (2 to 14) |
22 (7 to 37) |
34 (–173 to 240) |
||||||||||||
|
Aspirin |
–7 (–10 to –4) |
–11 (–19 to –4) |
138 (35 to 241) |
||||||||||||
|
Prescription analgesics |
|
|
|
||||||||||||
|
Tramadol |
–2 (–4 to 0) |
–1 (–7 to 6) |
–118 (–186 to –51) |
||||||||||||
|
Strong opioids |
30 (28 to 33) |
–34 (–41 to –26) |
–347 (–432 to –262) |
||||||||||||
|
NSAIDs |
–17 (–23 to –12) |
12 (–6 to 30) |
184 (–22 to 390) |
||||||||||||
|
Antimigraine medicines |
1 (1 to 2) |
0 (0 to 1) |
–1 (–10 to 9) |
||||||||||||
|
Gabapentinoids |
823 (65 to 102) |
–76 (–116 to –36) |
–803 (–1374 to –231) |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; NSAIDs = non‐steroidal anti‐inflammatory drugs. * The difference between the observed sales value and that expected were the pre‐announcement trend in sales maintained. † Including paracetamol/caffeine, paracetamol/diphenhydramine, paracetamol/metoclopramide. |
|||||||||||||||
Box 5 – Monthly sales to pharmacies of over‐the‐counter analgesic codeine‐containing products, 2015–2019, by tablets/capsules, packs, and kilograms
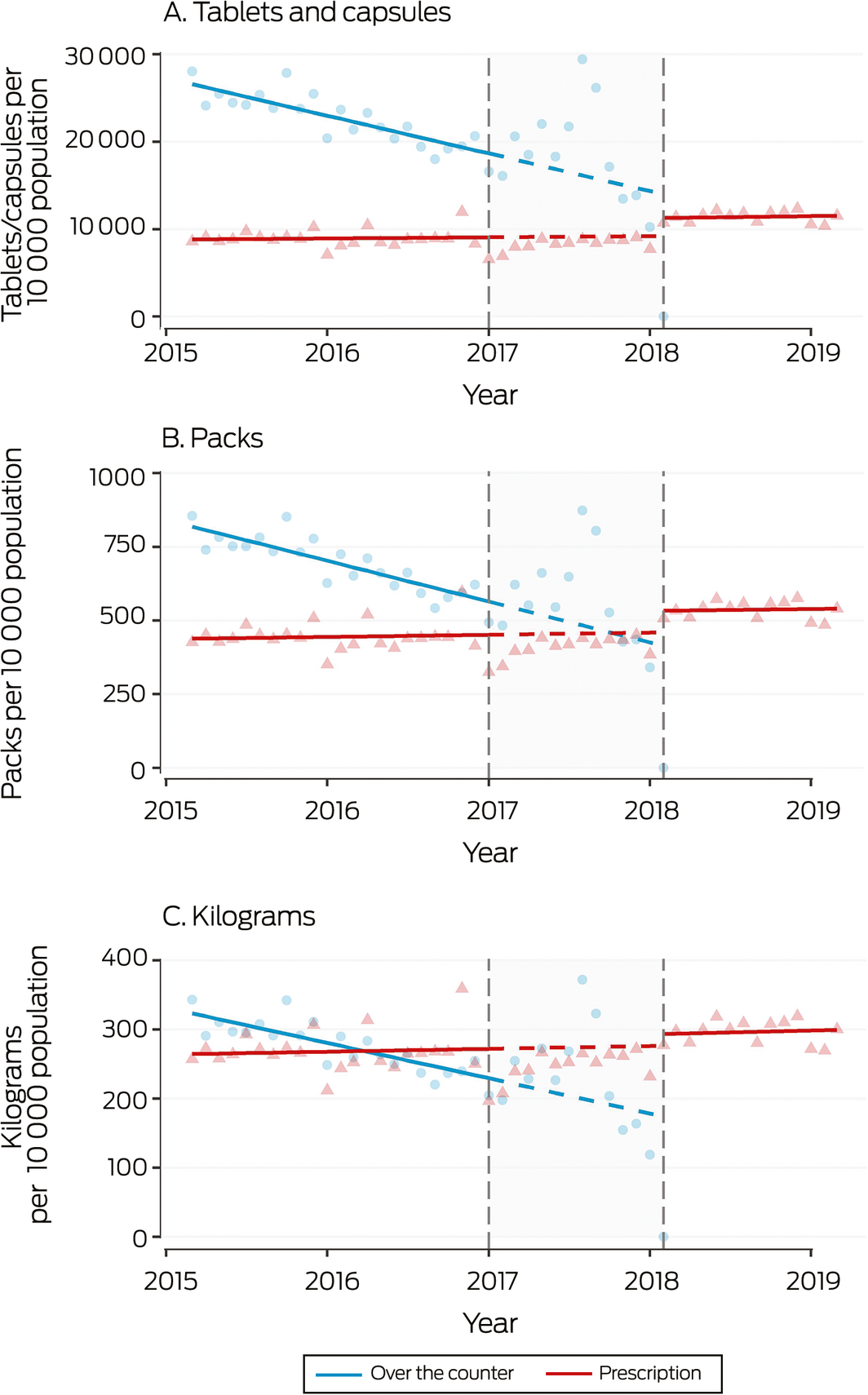
Shaded area: period between announcement (20 December 2016) and implementation (1 February 2018) of rescheduling of codeine‐containing products. Symbols: monthly values; solid line: fitted straight line trend; dashed line: continuation of pre‐announcement trend into period between announcement and implementation of rescheduling.
Box 6 – Monthly sales to pharmacies of over‐the‐counter analgesic products not including codeine, 2015–2019
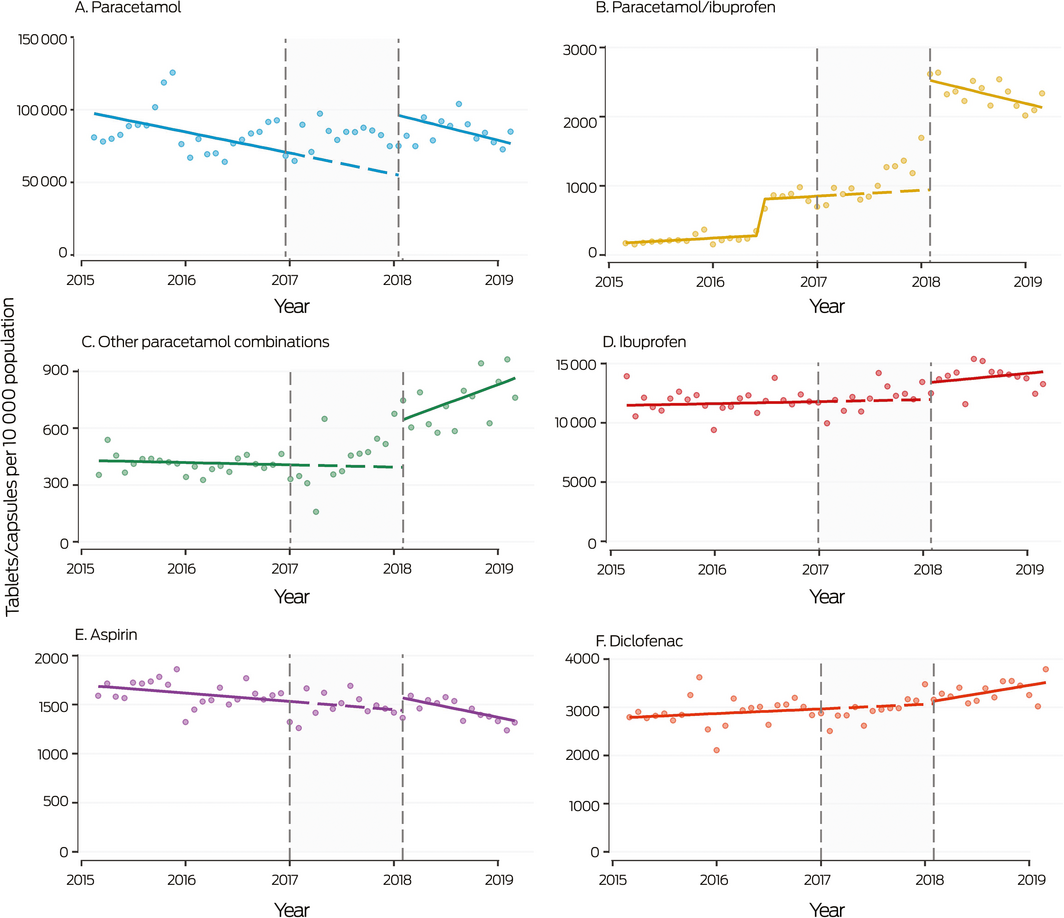
Shaded area: period between announcement (20 December 2016) and implementation (1 February 2018) of rescheduling of codeine‐containing products. Symbols: monthly values; solid line: fitted straight line trend; dashed line: continuation of pre‐announcement trend into period between announcement and implementation of rescheduling. The increase in paracetamol/ibuprofen sales in mid‐2016 was associated with the introduction of new products. The high rate of analgesic sales at the end of 2015 may reflect stockpiling of Pharmaceutical Benefits Scheme‐subsidised paracetamol before its delisting for the general population in January 2016.
Received 19 September 2019, accepted 18 December 2019
- Andrea L Schaffer1
- Rose Cairns2,3
- Jared A Brown1,3
- Natasa Gisev4
- Nicholas A Buckley2
- Sallie‐Anne Pearson1,5
- 1 Centre for Big Data Research in Health, University of New South Wales, Sydney, NSW
- 2 University of Sydney, Sydney, NSW
- 3 NSW Poisons Information Centre, Children's Hospital at Westmead, Sydney, NSW
- 4 National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW
- 5 Menzies Centre for Health Policy, University of Sydney, Sydney, NSW
This investigation was supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Medicines and Ageing (1060407). Andrea Schaffer is supported by an NHMRC Early Career Fellowship (1158763); Jared Brown is supported by a UNSW Scientia PhD Scholarship; and Natasa Gisev is supported by a UNSW Scientia Fellowship. We thank Billy Henderson (Mundipharma) for facilitating access to the IQVIA data. The IQVIA sales data were provided by Mundipharma under a third party licence; they were not involved in any aspect of the study.
Rose Cairns was an associate investigator on an untied educational grant from Seqirus for an investigation of tapentadol misuse. Jared Brown has received consultancy fees from GlaxoSmithKline Consumer for toxicology advice on paracetamol for an advisory board.
- 1. Gisev N, Nielsen S, Cama E, et al. An ecological study of the extent and factors associated with the use of prescription and over‐the‐counter codeine in Australia. Eur J Clin Pharmacol 2016; 72: 469–494.
- 2. Roxburgh A, Hall WD, Burns L, et al. Trends and characteristics of accidental and intentional codeine overdose deaths in Australia. Med J Aust 2015; 203: 299. https://www.mja.com.au/journal/2015/203/7/trends-and-characteristics-accidental-and-intentional-codeine-overdose-deaths.
- 3. Nielsen S, Roxburgh A, Bruno R, et al. Changes in non‐opioid substitution treatment episodes for pharmaceutical opioids and heroin from 2002 to 2011. Drug Alcohol Depend 2015; 149: 212–219.
- 4. Schug SA, Dobbin MD, Pilgrim JL. Caution with the forthcoming rescheduling of over‐the‐counter codeine‐containing analgesics [letter]. Med J Aust 2018; 208: 51–52. https://www.mja.com.au/journal/2018/208/1/caution-forthcoming-rescheduling-over-counter-codeine-containing-analgesics.
- 5. Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA 2017; 318: 1661–1667.
- 6. Daniels SE, Goulder MA, Aspley S, Reader S. A randomised, five‐parallel‐group, placebo‐controlled trial comparing the efficacy and tolerability of analgesic combinations including a novel single‐tablet combination of ibuprofen/paracetamol for postoperative dental pain. Pain 2011; 152: 632–642.
- 7. Cooper RJ. Over‐the‐counter medicine abuse: a review of the literature. J Subst Use 2013; 18: 82–107.
- 8. Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra‐rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J 2007; 7: 257–265.
- 9. Kelly LE, Rieder M, van den Anker J, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics 2012; 129: e1343–e1347.
- 10. Cairns R, Brown JA, Buckley NA. The impact of codeine re‐scheduling on misuse: a retrospective review of calls to Australia's largest poisons centre. Addiction 2016; 111: 1848–1853.
- 11. Therapeutic Goods Administration. Final decisions and reasons for decisions by delegates of the Secretary to the Department of Health. 25 Jan 2017. https://www.tga.gov.au/sites/default/files/scheduling-delegates-final-decision-codeine-december-2016.pdf (viewed Sept 2019).
- 12. Australian Medical Association. AMA backs TGA on codeine [media release]. 10 Aug 2017. https://ama.com.au/media/ama-backs-tga-codeine (viewed Aug 2019).
- 13. Royal Australian College of General Practitioners. Upscheduling of codeine. 2018. https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/upscheduling-of-codeine#advocacy (viewed July 2019).
- 14. Pharmacy Guild of Australia. Codeine upscheduling decision fails consumers. 20 Dec 2016. https://www.guild.org.au/news-events/news/2016/codeine-upscheduling-decision-fails-consumers (viewed July 2019).
- 15. Cairns R, Schaffer AL, Brown JA, et al. Codeine use and harms in Australia: evaluating the effects of re‐scheduling. Addiction 2020; 115: 451–459.
- 16. Australian Department of Health. Proposed listing changes for OTC items from 1 January 2016. http://www.pbs.gov.au/general/pbs-access-sustainability/otc-recommendations-for-1-january-2016.pdf (viewed Feb 2020).
- 17. Banken JA, Foster H. Dextromethorphan: an emerging drug of abuse. Ann N Y Acad Sci 2008; 1139: 402–411.
- 18. Australian Bureau of Statistics. 3101.0. Australian demographic statistics, Dec 2018. 19 Dec 2019. https://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/D56C4A3E41586764CA2581A70015893E?opendocument (viewed July 2019).
- 19. Bernal JL, Soumerai S, Gasparrini A. A methodological framework for model selection in interrupted time series studies. J Clin Epidemiol 2018; 103: 82–91.
- 20. Loboz KK, Shenfield GM. Drug combinations and impaired renal function: the “triple whammy”. Br J Clin Pharmacol 2005; 59: 239–243.
- 21. Frei MY, Nielsen S, Dobbin MDH, Tobin CL. Serious morbidity associated with misuse of over‐the‐counter codeine–ibuprofen analgesics: a series of 27 cases. Med J Aust 2010; 193: 294–296. https://www.mja.com.au/journal/2010/193/5/serious-morbidity-associated-misuse-over-counter-codeine-ibuprofen-analgesics.
- 22. Cairns R, Brown JA, Wylie CE, et al. Paracetamol poisoning‐related hospital admissions and deaths in Australia, 2004–2017. Med J Aust 2019; 211: 218–223. https://www.mja.com.au/journal/2019/211/5/paracetamol-poisoning-related-hospital-admissions-and-deaths-australia-2004-2017.
- 23. Haggan M. Media reports could have pushed stockpiling. AJP.com.au [internet], 25 Jan 2018. https://ajp.com.au/news/media-reports-pushed-stockpiling (viewed Aug 2019).
- 24. McCoy J, Bruno R, Nielsen S. Attitudes in Australia on the upscheduling of over‐the‐counter codeine to a prescription‐only medication. Drug Alcohol Rev 2018; 37: 257–261.





Abstract
Objective: To investigate changes in sales to pharmacies of over‐the‐counter (OTC) and prescription analgesics, cold and flu products, and cough suppressants after the rescheduling of codeine as a prescription only medicine in February 2018.
Design: Interrupted time series analysis of sales to pharmacies.
Setting: Pharmaceutical sales to community pharmacies in Australia, March 2015 – March 2019. The period January 2017 (month after rescheduling was announced) to January 2018 (month before rescheduling was implemented) was excluded from the time series analysis.
Main outcome measures: Monthly pack and tablet sales per 10 000 population of OTC and prescription analgesics, cold and flu products, and cough suppressants.
Results: During 2016, 7586 packs and 248 127 tablets of OTC codeine per 10 000 population were sold to pharmacies; in the 14 months after rescheduling, a small level increase in monthly prescription codeine sales was evident (2247 tablets/capsules per 10 000 population; 95% CI, 1231–3264 per 10 000 population). Monthly OTC analgesic sales increased by 258 (95% CI, 151–365) packs per 10 000 population and 37 856 (95% CI, 26 143–49 569) tablet/capsules per 10 000 population. Monthly sales of single ingredient paracetamol (41 415 [95% CI, 31 374–51 456] tablets/capsules per 10 000 population), ibuprofen (1392 [95% CI 916–1868] tablets/capsules per 10 000 population), paracetamol/ibuprofen (1618 tablets [95% CI, 1567–1669] tablets/capsules per 10 000 population), and other paracetamol combinations (233 [95% CI, 112–353] tablets/capsules per 10 000 population) all increased, but not those of prescription analgesic products not containing codeine. Rises for OTC cold/flu products containing the opioid derivative dextromethorphan were small; sales of OTC cough suppressants containing opioid derivatives (dextromethorphan, pholcodine, dihydrocodeine) did not change.
Conclusions: The rescheduling of codeine was followed by increased sales to pharmacies of paracetamol, ibuprofen, and paracetamol combination products. While these products carry no risk of dependence, their inappropriate use is also associated with harms that warrant adverse event monitoring.