Polycystic ovary syndrome (PCOS) affects 8–13% of reproductive age women, with around 21% of Indigenous women affected.1,2 Clinically, reproductive features are prominent and underpin PCOS diagnosis.3 The Rotterdam diagnostic criteria are based on two of three features: oligo- or anovulation, hyperandrogenism (clinical or biochemical) and polycystic ovaries on ultrasound, after exclusion of other causes (Box 1).3 Aetiology includes genetic causes, in utero hormone exposure and lifestyle factors (Box 2).4,5 PCOS is an endocrine disorder underpinned by insulin resistance and hyperandrogenism.5,6 It is associated with significant metabolic features including increased rates of gestational diabetes and type 2 diabetes mellitus as well as an increase in cardiovascular risk factors (Box 2).7 PCOS has significant psychological impact with increased depression and anxiety and impaired quality of life (Box 2).8,9 There is also an increased rate of weight gain and prevalence of obesity in PCOS, increasing severity of the condition, causing considerable concern for those affected and mandating attention to healthy lifestyle.10
Obtaining a timely PCOS diagnosis is challenging for women, with many experiencing delays with multiple different doctors involved.11-13 Inadequate information provision and lack of satisfaction with care has been reported, especially in areas such as psychological features, lifestyle and prevention. Doctors often focus on individual features of PCOS such as infertility, rather than taking a broader approach to care.13 There is also potential for overdiagnosis, including when isolated polycystic ovarian morphology on ultrasound is incorrectly equated with PCOS. Access to timely, accurate diagnosis and information provision needs significant improvement.
Clinically, there is considerable variation in care and evidence of confusion around diagnostic criteria.11,14 Researchers are frustrated with inadequate priority and funding in PCOS, especially given the prevalence, disease and economic burden.15 These challenges are exacerbated by inconsistent guideline quality and recommendations. Past guidelines have not followed recommended rigorous development processes, have not involved diverse health professionals including primary care providers, have not engaged women affected by PCOS, are country specific or, as in the case of the 2011 Australian PCOS guidelines,9 are now out of date. In this context, PCOS is a clear priority area for updated, consistent rigorously co-developed guidelines, translation resources and health professional and consumer support.16
The National Health and Medical Research Council (NHMRC) funded a Centre of Research Excellence to address current gaps. Through a multidisciplinary national alliance and international network, including primary care providers and women with PCOS, we have developed the International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018 and co-designed an extensive range of translation resources (Box 3).16
The guideline addresses:
-
screening, diagnostic assessment, risk assessment and life stage (Algorithm 1);
-
prevalence, screening, diagnostic assessment and treatment of emotional wellbeing (Algorithm 2);
-
lifestyle (Algorithm 3);
-
pharmacological treatment for non-fertility indications (Algorithm 4); and
-
assessment and treatment of infertility (Algorithm 5).
Diverse PCOS features are considered, including reproductive (hyperandrogenism, anovulation, infertility), metabolic (insulin resistance, impaired glucose tolerance, gestational and type 2 diabetes, adverse cardiovascular risk profiles) and psychological features (increased anxiety and depression and worsened quality of life).17 The guideline recognises variable presentation across the lifespan; ethnicity, which has an impact on dermatological and metabolic features; and cultural factors affecting experiences of women with PCOS.
The guideline abstract is provided in Box 4 and the full guideline is available at https://www.monash.edu/medicine/sphpm/mchri/pcos.
Guideline development process and methods
The guideline and translation program were developed through the integration of clinical perspectives, the preferences of women and the best available evidence. International society-nominated panels included consumers and experts in the fields of paediatrics, endocrinology, gynaecology, primary care, reproductive endocrinology, psychiatry, psychology, dietetics, exercise physiology, public health, project management and evidence synthesis translation. Governance included an international advisory and a project board, five guideline development groups, and consumer and translation committees. The NHMRC Australian Centre for Research Excellence in PCOS co-funded the guideline in partnership with the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine. Thirty-seven organisations across 71 countries collaborated to address 60 prioritised clinical questions based on 40 systematic and 20 narrative reviews, generating 166 recommendations. Methods met NHMRC standards and procedures for externally developed guidelines18 and are outlined in the full guideline and Box 5.19 These involved rigorous systematic review, training, online communication and face-to-face meetings to discuss the evidence and apply the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework. Evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength were agreed across the panel. Convened committees from partner and collaborating organisations provided peer review and the guideline was approved by the NHMRC.20-22
Women with PCOS and health professionals as the end users played a fundamental role in developing the guideline and resources. Stakeholder engagement and processes for guideline and translation resource development are outlined in Box 5.19 A summary of the recommendations is published elsewhere.20-22 The MJA has also published an editorial accompanying this supplement.23
In this supplement, we aim to optimise translation and implementation of the guideline (Box 4) by providing a brief clinical summary in the Australian context, noting key changes in practice, and by supporting health care providers, especially those in primary care, with co-designed, practical information, tools and consumer resources to improve outcomes and quality of life for women with PCOS (Box 3). We also highlight implications for Aboriginal and Torres Strait Islander women who are at high risk of PCOS.
The guideline is underpinned by a robust evaluation process which will enable practice benchmarking and feedback data to be collected to guide further alignment with evidence-based care. Downloads of the guideline and its resources will be monitored; focus groups and surveys will measure awareness in consumers and knowledge and practice change by health professionals. This information will then be compared with data that were collected before the guideline release, to measure change.
This strategic international approach involved strong partnership between consumers, multidisciplinary health professionals, academics and professional societies and we propose that this is transferable to other women’s health conditions and beyond. Such an approach can reduce duplication of effort and promote consistency of care and focus on provision of useful, accessible and meaningful resources to support both health professionals and people affected by a variety of conditions.24
Screening, diagnostic assessment, risk assessment and life stage (Algorithm 1)
We aim to improve accuracy and to simplify and facilitate timely diagnosis, while avoiding overdiagnosis, especially in adolescents. The guideline endorses the consensus-based Rotterdam diagnostic criteria3,20 for adult women and supports them with best available evidence. Algorithm 1 (https://www.monash.edu/__data/assets/pdf_file/0018/1411641/Algorithm-1-20180618.pdf) highlights the refined diagnostic criteria in adolescents, which require both hyperandrogenism and irregular cycles, with ultrasound now not recommended for diagnosis within 8 years of menarche, owing to overlap with normal reproductive physiology. Adolescents with clinical features but not a clear diagnosis are deemed “at risk”, with follow-up assessment recommended. Diagnostic features are refined, including irregular cycles, clinical and biochemical hyperandrogenism, and polycystic ovarian morphology. Exclusion of thyroid disease (thyroid-stimulating hormone), hyperprolactinemia (prolactin) and non-classic congenital adrenal hyperplasia (17-hydroxyprogesterone) is recommended, with further evaluation in patients with amenorrhea and more severe clinical features including consideration of hypogonadotropic hypogonadism, Cushing disease or androgen-producing tumours. The guideline recognises that PCOS is an insulin-resistant and metabolic disorder; tests for insulin resistance, however, lack accuracy and should not be incorporated into the diagnostic criteria for PCOS at this time. Anti-Müllerian hormone is likewise not recommended for diagnosis at this time.
Complication screening is recommended in PCOS. Cardiovascular risk factor screening is updated and simplified, including monitoring weight and weight change, assessing body mass index, family history, ethnicity, smoking status, blood pressure and glycaemic status in all patients with PCOS, and waist circumference and lipid profiles in those with additional risk factors. Frequency and type of testing are guided by presence of both PCOS and other risk factors. Obstructive sleep apnoea and endometrial cancer risk are also addressed.
Prevalence, screening, diagnostic assessment and treatment of emotional wellbeing (Algorithm 2)
Algorithm 2 (https://www.monash.edu/__data/assets/pdf_file/0004/1411645/Algorithm-2.pdf) highlights the increased prevalence of psychological features in PCOS, including anxiety and depressive symptoms, psycho-sexual dysfunction, eating disorders and disordered eating, and adverse impact on body image. The importance of these issues for women is recognised, along with the resulting significant impairment of quality of life. It is vital to ascertain and focus on the individual areas of most importance to women with PCOS. Appropriate screening is recommended based on risk, along with consideration of PCOS features that may have an impact on treatment.
In models of care, identification of priority areas for the individual with PCOS is paramount to enable targeted treatment that improves quality of life. Primary care providers are well positioned to assess, provide care and coordination and, if needed, refer to ensure that care is targeted to need and priority. Ethnic and cultural difference and life stage need consideration. Care should address short and long term psychological features and be mindful of how reproductive and metabolic features may affect psychological wellbeing.
Lifestyle (Algorithm 3)
Algorithm 3 (https://www.monash.edu/__data/assets/pdf_file/0008/1411649/Algorithm-3.pdf) highlights that healthy lifestyle and prevention of excess weight gain are critical for all women with PCOS from adolescence. Lifestyle interventions are similarly effective in women with and without PCOS, and are first line treatment in the majority of women with PCOS who are overweight. Modest weight loss of 5–10% of body weight is recommended to improve PCOS features, primarily through caloric restriction. No specific diet offers greater benefit in PCOS. Exercise recommendations are made for weight maintenance, health and weight loss. Incorporating behavioural strategies such as goal setting, self-monitoring, stimulus control, problem solving, assertiveness training, slower eating, reinforcing changes and relapse prevention are recommended to improve adherence and efficacy.
Pharmacological treatment for non-fertility indications (Algorithm 4)
Algorithm 4 (https://www.monash.edu/__data/assets/pdf_file/0019/1411651/Algorithm-4-20180801.pdf) outlines recommendations regarding pharmacological treatment for non-fertility indications. Combined oral contraceptive pills (COCPs) continue to be recommended as first line medical treatment for hyperandrogenism and regulation of menstrual cycles in PCOS. COCP efficacy is largely related to hepatic-mediated oestrogenic effects on sex hormone-binding globulin, which in turn decrease free testosterone levels. Other forms of hormonal contraception are less effective in this regard. COCP use for 6–12 months reduces androgens and hirsutism. Mood impacts have not been shown in PCOS; however, general population studies have noted COCP impact on libido and mood. No one preparation has been shown to be superior in PCOS, with all COCP agents increasing sex hormone-binding globulin and improving clinical outcomes. Based on general population data, COCPs are recommended at the lowest effective oestrogen dose balancing efficacy, metabolic risk profile, side effects, costs and availability, and 35 mg ethinyl estradiol preparations are not recommended first line treatment. Where COCPs and lifestyle changes fail in meeting treatment goals, metformin, as an insulin sensitiser, may assist in prevention of weight gain and improvement in metabolic features. Metformin improves body mass index, cyclicity, androgen levels and metabolic features, has demonstrated efficacy in combination with lifestyle modification, and is recommended in addition to lifestyle intervention, not as a substitute. Low dose therapy is recommended initially, with subsequent titration to reduce the mild and self-limiting gastrointestinal side effects. Anti-androgens in PCOS have limited evidence and are only recommended for hirsutism when at least 6 months of COCPs with cosmetic therapy have failed. Bariatric surgery can improve clinical features; however, registry studies demonstrate concerns around pregnancy outcomes and should only be considered in PCOS after lifestyle therapy fails.
Assessment and treatment of infertility (Algorithm 5)
Algorithm 5 (https://www.monash.edu/__data/assets/pdf_file/0003/1411653/Algorithm-5-20180619.pdf) outlines recommendations on pre-conception care and infertility management. While infertility assessment and management require specialist care, optimisation of psychological health, lifestyle intervention and provision of evidence-based resources to inform women on infertility treatment recommendations are well placed in primary care. The key change recommended in the guideline is the use of letrozole as first line pharmacological treatment in PCOS-associated and anovulatory infertility. Compared with clomiphene citrate, letrozole improves live birth rate, with a reduced rate of multiple pregnancy. However, in Australia, this involves off-label prescription and requires explanation and consent from the patient. Clomiphene citrate is recommended alone or combined with metformin. Metformin can be used alone, recognising lower success rates than other agents. When first line therapy has failed, exogenous gonadotrophins in women with clomiphene citrate-resistant PCOS are generally recommended second line, as is laparoscopic ovarian drilling. Importantly, in vitro fertilisation is indicated for women with PCOS and anovulation, after failure to respond to first and second line ovulation induction, or if there are other factors contributing to infertility. Algorithm 5 provides a detailed explanation regarding drug choice, based on clinical and other factors.
General practice tool, care plan and consumer resources to support care
Tools and resources have been developed with and for general practitioners, including a GP tool and care plan template to support decision making and evidence-based care in clinical practice (https://www.monash.edu/medicine/sphpm/mchri/pcos/resources/practice-tools-for-health-practitioners). A key component of the range of consumer tools, developed in partnership with consumers, is the PCOS app (AskPCOS). This is the first evidence-based PCOS app that has a range of unique features (eg, a comprehensive repository of information, question prompt list) to increase PCOS-related health literacy. GPs and other health professionals are the preferred source of consumer information, yet there is a clear need for complementary, additional, high quality, evidence-based resources for affected women. Such resources are accessible by searching “Monash PCOS” in your Web browser, or see Box 3 for direct links to the guideline resources for women and health professionals. These resources address demonstrated gaps, including inadequate and generally poor quality existing information, to support clinical care and improve knowledge, consumer engagement and health outcomes.
Considerations for Aboriginal and Torres Strait Islander women
Given the higher prevalence and more severe clinical features of PCOS in Aboriginal and Torres Strait Islander women,2,25 early diagnosis and management are vital to prevent and manage the reproductive, metabolic and psychological features of PCOS. This must be undertaken in a culturally appropriate and respectful manner.
In diagnosis, high quality ultrasound access may be challenging, as Aboriginal and Torres Strait Islander women are more likely to live in remote locations.26 The reduced guideline focus on ultrasound in PCOS diagnosis may assist here. Regarding screening for features of PCOS, Aboriginal and Torres Strait Islander women have increased risks of obesity, type 2 diabetes, dyslipidaemia and mental health disorders, independent of PCOS.27 They are more likely to be overweight or obese at all ages, with obesity contributing around 16% of the disparity in health burden.28 Diabetes is a key cause of mortality and disability-adjusted life-years in Aboriginal and Torres Strait Islander women,28 and PCOS amplifies these risks, making screening more critical.
Lifestyle management is the first line treatment for PCOS and associated complications. However, access to culturally appropriate care, services and lifestyle programs is suboptimal, because socio-economic factors, as well as lack of access to healthy food for those living in remote locations, create barriers to modifying lifestyle. Improving this situation is likely to require broader community and government action. There is little information about exercise among Aboriginal and Torres Strait Islander women. However, low participation rates in sports, less in women than men, have been noted.28 Facilitators of engagement include having a group, family, community or team focus, choice of activities and realistic goal attainment.29,30 Overall, we anticipate that the lifestyle recommendations in the guideline will be broadly applicable to Aboriginal and Torres Strait Islander women, but we acknowledge the socio-economic and geographical barriers and the need for adaptation to engage this population, especially in rural and remote locations.
Although the data are unclear, it is likely that infertility rates among Aboriginal and Torres Strait Islander women are at least similar to the national prevalence (the limited reports suggest they are actually higher) despite the younger age of first birth and increased total fertility rate.31,32 This emphasises the need for early diagnosis and for prevention. Socio-economic and geographic barriers may also affect uptake of medication recommendations (eg, letrozole) or procedures (eg, laparoscopic surgery). See also http://www.naccho.org.au/wp-content/uploads/1.National-guide-to-a-preventive-health-assessment-for-Aboriginal-and-Torres-Strait-Islander-people-2.pdf.
Resources are currently in the co-development phase with Aboriginal and Torres Strait Islander women.
Conclusion
The International evidence-based guideline on the assessment and management of polycystic ovary syndrome 2018 has been rigorously developed and informed by consumers, multidisciplinary health professionals and leading PCOS experts from six continents. The guideline is designed to address the needs of health professionals in providing better, timely care and improved outcomes for women with PCOS, as well as enabling women to be more informed and involved in their treatment.
The PCOS guideline and translation resources aim to accelerate the delivery of consistent, evidence-based care across Australia. GPs are well supported in the implementation of the recommendations from the PCOS guideline by the provision of the range of freely available practice tools, tailored to the Australian context. GPs can also augment the PCOS-related health literacy of consumers by directing them to the range of consumer resources.
Box 2 – Aetiology and clinical manifestations of polycystic ovary syndrome
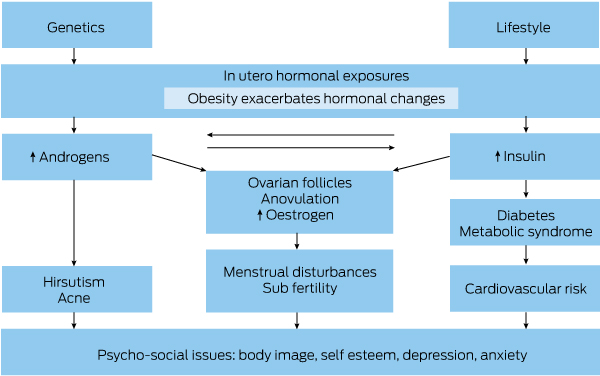
Adapted from Teede et al.9
Box 3 – Resources for women and health professionals to support evidence-based care in polycystic ovary syndrome (PCOS)
|
|
|||||||||||||||
|
Resources for health professionals |
|||||||||||||||
|
Algorithm 1 |
Screening, diagnostic assessment, risk assessment and life stage |
||||||||||||||
|
Algorithm 2 |
Prevalence, screening, diagnostic assessment and treatment of emotional wellbeing |
||||||||||||||
|
Algorithm 3 |
Lifestyle |
||||||||||||||
|
Algorithm 4 |
Pharmacological treatment for non-fertility indications |
||||||||||||||
|
Algorithm 5 |
Fertility treatment |
||||||||||||||
|
Online programs |
Webinars, interviews with experts and women with PCOS |
||||||||||||||
|
Certificate programs |
Online (fee-paying education programs) |
||||||||||||||
|
GP Tool and care plan |
Outline of PCOS for GPs to be used during the consultation |
||||||||||||||
|
Resources for women |
|||||||||||||||
|
Infographic 1 |
What is PCOS and do I have it? |
||||||||||||||
|
Infographic 2 |
Lifestyle and PCOS |
||||||||||||||
|
Infographic 3 |
Emotional wellbeing and PCOS |
||||||||||||||
|
Infographic 4 |
PCOS medical treatment |
||||||||||||||
|
Infographic 5 |
Fertility and PCOS |
||||||||||||||
|
Video series |
A series of videos delivered by experts on all aspects of PCOS |
||||||||||||||
|
Mobile app |
Available on Apple iTunes and Google platforms — multilingual options in development |
||||||||||||||
|
Written resources |
Fact sheets, e-resources, booklets and tools available online |
||||||||||||||
|
Online programs |
Webinars and online resources and presentations |
||||||||||||||
|
Question prompt list |
Question list embedded in the app or available stand-alone to improve interaction between women with PCOS and their health professionals |
||||||||||||||
|
|
|||||||||||||||
|
All resources are available at https://www.monash.edu/medicine/sphpm/mchri/pcos |
|||||||||||||||
Box 4 – Abstract from the International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018
Objective: To develop and translate rigorous, comprehensive evidence-based diagnosis, assessment and treatment guidelines, to improve the lives of women with polycystic ovary syndrome (PCOS) worldwide.
Participants: Extensive health professional and patient engagement informed guideline priority areas. International Society-nominated panels included consumers, paediatrics, endocrinology, gynaecology, primary care, reproductive endocrinology, psychiatry, psychology, dietetics, exercise physiology, public health, project management, evidence synthesis and translation experts.
Evidence: Best practice evidence-based guideline development involved extensive evidence synthesis and the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework covered evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength.
Process: Governance included an international advisory board from six continents, a project board, five guideline development groups with 63 members, consumer and translation committees. The Australian Centre for Research Excellence in PCOS, funded by the National Health and Medical Research Council (NHMRC), partnered with European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine. Thirty seven organisations across 71 countries collaborated with 23 face to face international meetings over 15 months. Sixty prioritised clinical questions involved 40 systematic and 20 narrative reviews, generating 166 recommendations and practice points. Convened Committees from partner and collaborating organisations provided peer review and the guideline was approved by the NHMRC.
Conclusions: We endorse the Rotterdam PCOS diagnostic criteria in adults (two of clinical or biochemical hyperandrogenism, ovulatory dysfunction, or polycystic ovaries on ultrasound) and where irregular menstrual cycles and hyperandrogenism are present, highlight that ultrasound is not necessary in diagnosis. Within eight years of menarche, both hyperandrogenism and ovulatory dysfunction are required, with ultrasound not recommended. Ultrasound criteria are tightened with advancing technology. Anti-Müllerian hormone levels are not yet adequate for diagnosis. Once diagnosed, assessment and management includes reproductive, metabolic and psychological features. Education, self-empowerment, multidisciplinary care and lifestyle intervention for prevention or management of excess weight are important. Depressive and anxiety symptoms should be screened, assessed and managed with the need for awareness of other impacts on emotional wellbeing. Combined oral contraceptive pills are firstline pharmacological management for menstrual irregularity and hyperandrogenism, with no specific recommended preparations and general preference for lower dose preparations. Metformin is recommended in addition or alone, primarily for metabolic features. Letrozole is first-line pharmacological infertility therapy; with clomiphene and metformin having a role alone and in combination. In women with PCOS and anovulatory infertility, gonadotrophins are second line. In the absence of an absolute indication for IVF, women with PCOS and anovulatory infertility, could be offered IVF third line where other ovulation induction therapies have failed. Overall evidence is low to moderate quality, requiring significant research expansion in this neglected, yet common condition. Guideline translation will be extensive including a multilingual patient mobile application and health professional training.
Reproduced with permission from the author Helena Teede on behalf of Monash University, which holds the copyright: https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS-Evidence-Based-Guideline.pdf
Box 5 – Guideline development process

GDG = guideline development group. *Time points and tasks where prioritisation of engagement from GDG is required.
Reproduced with permission from Misso and Teede.19
Provenance: Commissioned; externally peer reviewed.
- Helena J Teede1,2
- Marie L Misso1,2
- Jacqueline A Boyle1,2
- Rhonda M Garad1,2
- Veryan McAllister1,3
- Linda Downes1,2
- Melanie Gibson-Helm1,2
- Roger J Hart1,4
- Luk Rombauts5
- Lisa Moran1,2
- Anuja Dokras6
- Joop Laven7
- Terhi Piltonen8
- Raymond J Rodgers1,9
- Mala Thondan10
- Michael F Costello1,11
- Robert J Norman1,9
- on behalf of the International PCOS Network
- 1 National Health and Medical Research Council Centre for Research Excellence in PCOS, Monash and Adelaide Universities, Melbourne, VIC
- 2 Monash Centre for Health Research and Implementation, Monash Public Health and Preventive Medicine, Monash University and Monash Health, Melbourne, VIC
- 3 Polycystic Ovary Syndrome Association of Australia, Sydney, NSW
- 4 University of Western Australia, Perth, WA
- 5 Department of Obstetrics and Gynaecology, Monash University, Melbourne, VIC
- 6 Obstetrics and Gynecology, University of Pennsylvania, Philadelphia, PA, USA
- 7 Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynaecology, Erasmus Medical Centre, Rotterdam, Netherlands
- 8 Obstetrics and Gynecology, PEDEGO Research Unit, Medical Research Centre, Oulu University Hospital, Oulu, Finland
- 9 Robinson Research Institute, University of Adelaide and Fertility SA, Adelaide, SA
- 10 Harp Family Medical Centre, Melbourne, VIC
- 11 UNSW Sydney, Sydney, NSW
Collaborating authors: Estifanos Baye, Monash Centre for Health Research and Implementation, Melbourne; Leah Brennan, Australian Catholic University, Melbourne; Cheryce Harrison, Monash Centre for Health Research and Implementation, Melbourne; Samantha Hutchison, Monash Health Centre for Research Implementation, Melbourne; Anju Joham, Monash Centre for Health Research and Implementation, Melbourne; Louise Johnson, Victorian Assisted Reproductive Treatment Authority, Melbourne; Cailin Jordan, Genea Hollywood Fertility, Perth; Jayashri Kulkarni, Monash Alfred Psychiatry Research Centre, Melbourne; Darren Mansfield, Monash Health, Melbourne; Kate Marsh, Northside Nutrition and Dietetics, Sydney; Ben W Mol, Monash University, Melbourne; Alexia Peña, Robinson Research Institute, University of Adelaide, Adelaide; Raymond Rodgers, Robinson Research Institute, University of Adelaide, Adelaide; Jane Speight, Deakin University, Geelong; Nigel Stepto, Victoria University, Melbourne; Eliza C Tassone, Monash Centre for Health Research and Implementation, Melbourne; Angela Wan, Monash University, Melbourne; Jane Woolcock, Women’s and Children’s Hospital, Adelaide.
We gratefully acknowledge the contribution of the many women with PCOS and health professionals who guided and contributed to this work. We thank our funding, partner, engaged and collaborating organisations for their roles in prioritising topics and identifying gaps, and contributing members for guideline development, providing peer review and assisting with dissemination. We acknowledge those who independently assessed the guideline against AGREEII criteria and completed methodological review, and those within the NHMRC who managed the approval process. This guideline was approved by all members of the guideline development groups and has been approved by the NHMRC.
Specifically, our funding, partner, collaborator and engaged organisations include:
• The NHMRC through the funded Centre for Research Excellence in Polycystic Ovary Syndrome and the members of this Centre who coordinated this international guideline effort.
• Our partner organisations which co-funded the guideline: the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology.
• Our collaborating and engaged societies and consumer groups: Androgen Excess and Polycystic Ovary Syndrome Society; American Pediatric Endocrine Society; Asia Pacific Paediatric Endocrine Society; Asia Pacific Initiative on Reproduction; Australasian Paediatric Endocrine Group; Australian Diabetes Society; British Fertility Society; Canadian Society of Endocrinology and Metabolism; Dietitians Association Australia; Endocrine Society (US);Endocrine Society Australia; European Society of Endocrinology; European Society for Paediatric Endocrinology; Exercise and Sports Science Australia; Fertility Society Australia; International Society of Endocrinology; International Federation of Fertility Societies; International Federation of Gynaecology and Obstetrics; Italian Society of Gynaecology and Obstetrics; Japanese Society for Paediatric Endocrinology; Latin American Society for Paediatric Endocrinology; Nordic Federation of Societies of Obstetrics and Gynaecology; PCOS Challenge; PCOS Society of India; Paediatric Endocrine Society; Polycystic Ovary Association Australia; Royal Australasian College of Physicians; Royal Australian College of General Practitioners; Royal Australian and New Zealand College of Obstetricians and Gynaecologists; Royal College of Obstetricians and Gynaecologists (UK); South African Society of Gynaecology and Obstetrics; Verity UK; Victorian Assisted Reproductive Technology Association (VARTA).
• Our Australian translation partners: Jean Hailes for Women’s Health and VARTA.
Funding: The guideline and translation program was primarily funded by the NHMRC Centre for Research Excellence in PCOS grant (APP1078444) and partnership grant (APP1133084). This funding was supported by a partnership with the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine. Translation costs were supported by the NHMRC Centre for Research Excellence and partnership grant. Jean Hailes for Women’s Health funded the cost of this MJA supplement.
Disclosures of conflicts of interest were declared at the outset and updated throughout the guideline process, aligned with NHMRC guideline processes. Full details of conflicts declared across the guideline development groups are available at guideline in the register of disclosures of interest.
- 1. Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod 2016; 31: 2841-2855.
- 2. Boyle JA, Cunningham J, O’Dea K, et al. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust 2012; 196: 62-66. <MJA full text>
- 3. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum Reprod 2004; 19: 41-47.
- 4. Tata B, Mimouni NEH, Barbotin A-L, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med 2018; 24: 834-846.
- 5. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33: 981-1030.
- 6. Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 2013; 28: 777-784.
- 7. Moran LJ, Norman RJ, Teede HJ. Metabolic risk in PCOS: phenotype and adiposity impact. Trends Endocrinol Metab 2015; 26: 136-143.
- 8. Dokras A, Stener-Victorin E, Yildiz BO, et al. Androgen excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril 2018; 109: 888-899.
- 9. Teede HJ, Misso ML, Deeks AA, et al. Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust 2011; 195 (6 Suppl): S65-S110. <MJA full text>
- 10. Teede HJ, Joham AE, Paul E, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity 2013; 21: 1526-1532.
- 11. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017; 102: 604-612.
- 12. Teede H, Gibson-Helm M, Norman RJ, et al. Polycystic ovary syndrome: perceptions and attitudes of women and primary health care physicians on features of PCOS and renaming the syndrome. J Clin Endocrinol Metab 2014; 99: E107-E111.
- 13. Gibson-Helm ME, Lucas IM, Boyle JA, Teede HJ. Women’s experiences of polycystic ovary syndrome diagnosis. Fam Pract 2014; 31: 545-549.
- 14. Dokras A, Saini S, Gibson-Helm M, et al. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil Steril 2017; 107: 1380-1386.e1.
- 15. Brakta S, Lizneva D, Mykhalchenko K, et al. Perspectives on Polycystic Ovary Syndrome: Is Polycystic Ovary Syndrome Research Underfunded? J Clin Endocrinol Metab 2017; 102: 4421-4427.
- 16. Teede H, Legro R, Norman R. A vision for change in PCOS through international collaboration. Semin Reprod Med 2018; in press.
- 17. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010; 8: 41.
- 18. National Health and Medical Research Council. 2016 NHMRC Standards for Guidelines. https://www.nhmrc.gov.au/guidelines-publications/information-guideline-developers/2016-nhmrc-standards-guidelines (viewed Aug 2018).
- 19. Misso M, Teede H. Evidence based guideline (EBG) development: a practical guide. In: Ilic D, editor. Knowledge transfer: practices, types and challenges. New York: Nova Publishers, 2012.
- 20. Teede HJ, Misso ML, Costello MF, at al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018; 110: 364-379.
- 21. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2018; doi:10.1111/cen.13795[Epub ahead of print].
- 22. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018; doi:10.1093/humrep/dey256 [Epub ahead of print].
- 23. Norman R, Teede H. A new evidence-based guideline for assessment and management of polycystic ovary syndrome. Med J Aust 2018; 209: 299-300.
- 24. National Institutes of Health. NIH evidence-based methodology workshop on Polycystic Ovary Syndrome: executive summary. 3-5 Dec 2012. https://prevention-archive.od.nih.gov/docs/programs/pcos/FinalReport.pdf (viewed Aug 2018).
- 25. Davis SR, Knight S, White V, et al. Preliminary indication of a high prevalence of polycystic ovary syndrome in indigenous Australian women. Gynecol Endocrinol 2002; 16: 443-446.
- 26. Boyle J, Hollands G, Beck S, et al. Process evaluation of a pilot evidence-based Polycystic Ovary Syndrome clinic in the Torres Strait. Aust J Rural Health 2017; 25: 175-181.
- 27. Australian Bureau of Statistics. 4715.0 National Aboriginal and Torres Strait Islander Health Survey, 2004-05. Canberra: ABS, 2006. http://www.abs.gov.au/ausstats/abs@.nsf/mf/4715.0/ (viewed Jan 2018).
- 28. Vos T, Barker B, Begg S, et al. Burden of disease and injury in Aboriginal and Torres Strait Islander Peoples: the Indigenous health gap. Int J Epidemiol 2009; 38: 470-477.
- 29. Hunt J, Marshall AL, Jenkins D. Exploring the meaning of, the barriers to and potential strategies for promoting physical activity among urban Indigenous Australians. Health Promot J Aust 2008; 19: 102-108.
- 30. Thompson SJ, Gifford SM, Thorpe L. The social and cultural context of risk and prevention: food and physical activity in an urban Aboriginal community. Health Educ Behav 2000; 27: 725-743.
- 31. Hancock H. Aboriginal women’s perinatal needs, experiences and maternity services: a literature review to enable considerations to be made about quality indicators. Ngaanyatjarra Health Service, Dec 2006. https://www.lowitja.org.au/sites/default/files/docs/Ngaanyatjarra-Health-Service-Lit-Review.pdf (viewed Aug 2018).
- 32. Kildea S, Bowden FJ. Reproductive health, infertility and sexually transmitted infections in Indigenous women in a remote community in the Northern Territory. Aust N Z J Public Health 2000; 24: 382-386.






Abstract
Introduction: We have developed the first international evidence-based guideline for the diagnosis and management of polycystic ovary syndrome (PCOS), with an integrated translation program incorporating resources for health professionals and consumers. The development process involved an extensive Australian-led international and multidisciplinary collaboration of health professionals and consumers over 2 years. The guideline is approved by the National Health and Medical Research Council and aims to support both health professionals and women with PCOS in improving care, health outcomes and quality of life. A robust evaluation process will enable practice benchmarking and feedback to further inform evidence-based practice. We propose that this methodology could be used in developing and implementing guidelines for other women’s health conditions and beyond.
Main recommendations: The recommendations cover the following broad areas: diagnosis, screening and risk assessment depending on life stage; emotional wellbeing; healthy lifestyle; pharmacological treatment for non-fertility indications; and assessment and treatment of infertility.
Changes in management as a result of this guideline: •Diagnosis:▪when the combination of hyperandrogenism and ovulatory dysfunction is present, ultrasound examination of the ovaries is not necessary for diagnosis of PCOS in adult women;▪requires the combination of hyperandrogenism and ovulatory dysfunction in young women within 8 years of menarche, with ultrasound examination of the ovaries not recommended, owing to the overlap with normal ovarian physiology; and▪adolescents with some clinical features of PCOS, but without a clear diagnosis, should be regarded as “at risk” and receive follow-up assessment.•Screening for metabolic complications has been refined and incorporates both PCOS status and additional metabolic risk factors.•Treatment of infertility: letrozole is now first line treatment for infertility as it improves live birth rates while reducing multiple pregnancies compared with clomiphene citrate.