The known Microvascular complications in people with type 1 diabetes mellitus are directly related to glycaemic control.
The new This is the first study to assess the risk of complications in people with type 1 diabetes according to their glycaemic control trajectory between childhood and adulthood. Severe diabetic retinopathy (SDR) was associated with higher paediatric HbA1c levels, independent of glycaemic control during adulthood. Importantly, SDR was not documented in patients with a stable low glycaemic control trajectory.
The implications Target-based treatment from the time of diagnosis of type 1 diabetes in childhood is required to reduce the risk of SDR during adulthood.
Whether microvascular complications develop in people with type 1 diabetes mellitus is critically dependent on their glycaemic control.1-3 In the large Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Complications (EDIC) trial, however, mean glycated haemoglobin A1c (HbA1c) levels could only be estimated from data acquired at trial entry; consequently, the effect of the cumulative glycaemic exposure of the 195 adolescents in these studies during their 1–5 years of diabetes could not be analysed. As a result, the importance and contribution of childhood glycaemic control could not be fully assessed, which may explain some of the differences between adolescent and adult outcomes at follow-up.4 Apart from these two large scale studies, few investigations have followed individuals with childhood-onset type 1 diabetes into adult life.5,6 One longitudinal study (15 participants) found that mean HbA1c levels at diagnosis in childhood were higher for those who developed retinopathy during the 20-year follow-up, and that differences in HbA1c levels between those with and without retinopathy gradually declined with time.7 However, no study has compared the effect of optimal and poor glycaemic control across life on the risk of later complications.
The objectives of our study were to examine the impact of childhood glycaemic control on the future risk of complications in people with type 1 diabetes. Specifically, we aimed to delineate the effect of glycaemic control trajectory on risk, and to determine the relative effects of paediatric and adult metabolic control. We hypothesised that a stable low trajectory would be associated with a lower risk of microvascular complications, and that glycaemic control during childhood would modify the future risk of complications.
Methods
Study design
We undertook a retrospective cohort study of data collected from the time of diagnosis of type 1 diabetes in childhood until the time of our analysis (November 2013). Adults with a diagnosis of type 1 diabetes8 (diagnosed in childhood during 1975–2010) were included if they had attended at least one specialist adult diabetes clinic at the Royal Melbourne Hospital, and their care had been transferred from the paediatric diabetes clinic at the Royal Children’s Hospital (Melbourne) during 1992–2013. Individuals who had been lost to follow-up at the time of care transition from the paediatric diabetes clinic or who had died were therefore excluded. The choice of transition referral centre follows a discussion between the physician and young adult, and is not based on any biological or clinical criteria. Because of its proximity, the Royal Melbourne is the main adult referral centre for patients who transition from the Royal Children’s Hospital, receiving about 40% of its transitioning cohort.
We used a data linkage system, BioGrid Australia, that facilitates linkage of de-identified clinical data from member institutions. All individuals with type 1 diabetes common to both hospitals were identified. Data obtained from clinical department databases at each institution, including standardised clinical data for all routine outpatient clinic visits, were combined with mortality outcome data from the National Death Index (NDI), which has recorded all deaths in Australia since 1980. The process of sequential data linkage was performed with SAS 9.2 (SAS Institute).
Main outcomes and measures
Severe complications
The primary outcome of interest was a database record of diabetes-specific microvascular complications; in this study, only the most severe forms were considered. The date and cause of death were obtained from the NDI. Severe diabetic retinopathy (SDR) included one or more of maculopathy, proliferative retinopathy, and a need for photocoagulation surgery. Chronic kidney disease (CKD) was defined by a glomerular filtration rate of less than 60 mL/min/1.73 m2 (stage 3 CKD or worse),9 calculated from serial creatinine measurements using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.10 Ulceration and amputation were recorded according to the clinical database files.
Glycaemic control
HbA1c levels were summarised as paediatric (mean of all pre-transition paediatric clinic measurements), adult (mean of all post-transition measurements as an adult), and life course values (mean HbA1c level from diagnosis to November 2013). The glycaemic control trajectory was defined across the life course, with 66 mmol/mol set as the upper cut-off value for good glycaemic control. This value was preferred to the standard paediatric target of 58 mmol/mol because it was anticipated that some of the cohort had commenced treatment before publication of the DCCT findings1,2 upon which the current HbA1c target values are based.8,11 The median HbA1c level in children aged 0–18 years with type 1 diabetes in Australia in 2009 was reported to be 66 mmol/mol;12 this was also the median HbA1c level for a cohort of children who had recently transitioned from care at the Royal Children’s Hospital.13
Each individual was assigned to one of four glycaemic control trajectory groups:
-
stable low (mean paediatric and adult HbA1c ≤ 66 mmol/mol);
-
improving (mean paediatric HbA1c > 66 mmol/mol, mean adult HbA1c ≤ 66 mmol/mol);
-
worsening (mean paediatric HbA1c ≤ 66 mmol/mol, mean adult HbA1c > 66 mmol/mol); or
-
stable high (paediatric and adult mean HbA1c > 66 mmol/mol).
Statistical analyses
Differences between the trajectory groups in participant characteristics, HbA1c levels, and complications were examined by one-way ANOVA (continuous variables) or in χ2 tests (categorical variables). The standardised mortality ratio was calculated as the ratio of the number of observed deaths to the number of expected deaths in the general population, based on 2012 Australian Bureau of Statistics data for Victoria. SDR was the only complication we examined in a regression analysis, as the aetiology of the other outcome measures could not be precisely defined. The relative effect of paediatric and adult glycaemic control on the risk of developing SDR was assessed by generalised estimating equation (GEE) analysis, which could allow for unmeasured variables and confounders. Statistical analyses were performed in Stata 13.0 (StataCorp); P < 0.05 was deemed statistically significant.
Ethics approval
The study received ethics approval from all participating institutions, the Royal Children’s Hospital Human Research Ethics Committee (reference, 31206), BioGrid (project reference, 201202/1), and the Australian Institute of Health and Welfare Research Ethics Committee (reference, EC2013-2-30).
Results
Participant characteristics
We identified 503 people (including 253 men) who were diagnosed with type 1 diabetes during 1975–2010 and had transitioned from paediatric to adult diabetes services over a 21-year period (1992–2013) at a mean age of 18.4 years (standard deviation [SD], 0.9 years; Box 1). The mean age at diagnosis was lower for girls (9.6 [SD, 3.9] v 10.3 [SD, 4.1] years; P < 0.05) but higher for women at the time of our analysis (28.8 [SD, 6.7] v 27.2 [SD, 5.7] years; P < 0.05); the mean duration of type 1 diabetes was therefore longer for women (19.3 [SD, 7.8] v 16.9 [SD, 7.1] years; P < 0.01). The mean number of HbA1c measurements per individual was 22.0 (SD, 13.0), 10.0 (SD, 8.1) and 29.6 (SD, 15.9) during the paediatric, adult and life course periods respectively; the corresponding mean HbA1c levels were 68 mmol/mol (SD, 13.1), 70 mmol/mol (SD, 17.5), and 68 mmol/mol (SD, 12.0) (Box 1).
Severe complications
At least one severe complication was documented for 26 participants (5.2%), including 16 with SDR (3.2%; Box 1). No severe complications were recorded in the paediatric dataset. Based on age- and sex-matched data from 2012 Victorian state data, the overall standardised mortality ratio in this cohort was 1.9 (95% CI, 0.7–4.3) (men, 1.3 [95% CI, 0.2–4.1]; women, 2.7 [95% CI, 0.7–7.4]).
Lifetime glycaemic control trajectory and risk of complications
For the stable low group (143 participants, 28%), the mean paediatric, adult and overall HbA1c levels were 57 mmol/mol (SD, 6.6), 57 mmol/mol (SD, 6.6), and 58 mmol/mol (SD, 3.3) respectively (Box 1). Only one person in this group had a documented complication (a 29-year-old man who had had an amputation).
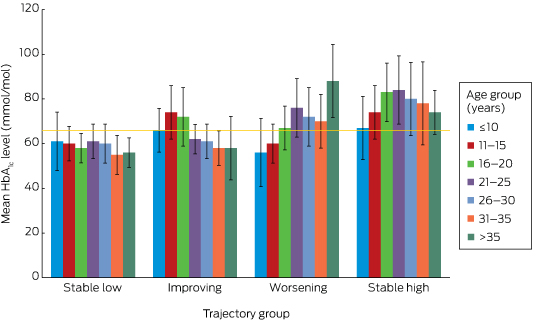
The glycaemic profiles for the stable low, improving (82 participants, 16%), worsening (96 participants, 19%) and stable high trajectories (182 participants, 36%) are shown in Box 2. Given the low frequency of complications, further analyses were restricted to SDR, for which a causative role for hyperglycaemia could be confidently assumed. No-one in the stable low group had developed SDR, but three in the improving (4%), one in the worsening (1%), and 12 in the stable high groups (7%) had developed SDR (P = 0.004; Box 1). The overall mean age of onset of SDR was 28.8 years (SD, 4.4) years (for the improving group, 23.9 years [SD, 3.7]; worsening group, 28.5 years; stable high, 30.3 years [SD, 3.9]; P = 0.6). However, the mean interval between diagnosis with type 1 diabetes and onset of SDR was shorter for the worsening (30.5 years) and stable high groups (28.1 years; SD, 0.8) than for the improving group (31.9 years; SD, 6.2; P = 0.01).
Paediatric HbA1c level and SDR risk in adulthood
GEE analysis that included significant variables from exploratory multivariate logistic regression models (online Appendix) indicated that each 10.9 mmol/mol increase in paediatric HbA1c level was associated with an almost threefold risk of SDR (odds ratio [OR], 2.9; 95% CI, 1.9–4.3; P < 0.01); each 10.9 mmol/mol increase in adult HbA1c level was associated with a twofold risk (OR, 2.1; 95% CI, 1.4–3.1; P < 0.01). Longer duration of type 1 diabetes was also associated with an increased risk of SDR (per additional year: OR, 1.3; 95% CI, 1.2–1.5; P < 0.01).
Discussion
By incorporating all recorded HbA1c data from diagnosis onwards, this study offers a unique insight into a cohort of adults with childhood-onset type 1 diabetes who were not managed in clinical trials. None of those who maintained a mean HbA1c level of 66 mmol/mol or less from the time of diagnosis (the stable low group) developed SDR. The mean paediatric, adult and overall HbA1c levels in this group were each 58 mmol/mol or less, supporting the adoption of this target in paediatric practice.11 Each additional year of diabetes conferred a significant increase in the risk of SDR, and our data indicate that both paediatric and adult mean HbA1c levels are modifiable factors that moderate this risk. This is important for paediatric care providers, as 64.6% of participants remained in the same HbA1c level category (low or high) during the paediatric and adult periods, indicating that glycaemic control generally neither markedly deteriorates nor improves after the transition to adult services. This challenges the widely held belief that glycaemic control in young adults with type 1 diabetes improves during their mid- to late 20s following deterioration during the adolescent years,14 a premise that may not apply to every patient.
The major limitations of this study are its retrospective design and the low numbers of severe complications reported. Detailed clinical information beyond that recorded in the clinical databases was not available; as the data were de-identified, this problem could not be overcome. Assessing the potential relevance of lifetime glycaemic control for the risk of complications, with the exception of retinopathy, is therefore difficult. Further, we lacked information about outcomes for those who were lost to follow-up immediately after the transition from paediatric care, for whom we consequently have no information about glycaemic control trajectory or complication rates. This could account for the discrepancy between the standardised mortality ratio we estimated and that based on a population-based dataset in Western Australia (1.7 for men, 10.1 for women).15 Although glycaemic control for most of the participants had been suboptimal throughout their lives, the SDR rate was low, but consistent with the recent report that 3.7% of young people (14–30 years old) with type 1 diabetes in Norway required laser therapy within 20 years of the onset of diabetes.16
A number of factors contribute to a higher risk of diabetes-related complications, including genetic susceptibility and cardiovascular risk factors (such as smoking, higher body mass index, greater waist:hip ratio, hyperlipidaemia, hypertension). Data on these factors were not available, and the omission of these known confounders from our analyses is a major limitation of this study. The duration of follow-up varied between individuals, and a shorter period of follow-up during adulthood may have led to misclassification of trajectory category. Cohort studies that assess individuals from diagnosis to death could overcome this limitation, but would be possible only for population-based registries or in large, multicentre cohort studies.
As the study period was broad, we also assessed the effect of era of diagnosis on SDR outcome (data not shown). While SDR was more common among those diagnosed prior to the publication of the DCCT findings (1994), the effect was not independent of the collinear higher glycaemic control that commenced before contemporary target-based practice.
Our report describes the risk of diabetes-specific microvascular complications in a cohort of Australian adults who were diagnosed with type 1 diabetes during childhood. It is the first to assess clinical outcomes according to glycaemic control trajectory between childhood and adulthood, and is the largest to use all available metabolic data from the diagnosis of type 1 diabetes onwards, with a longer duration of follow-up than reported elsewhere. In the absence of an Australian population-based registry of individuals with type 1 diabetes, this data linkage study facilitated assessment of the effects of glycaemic control during the paediatric and adult periods. From this novel perspective, we found that, after adjusting for duration of diabetes (a non-modifiable factor), HbA1c level throughout the course of life was independently associated with the risk of retinopathy in adulthood; the predictive effects of paediatric and adult HbA1c levels were equivalent. However, as severe retinopathy commenced during the third decade of life in our cohort and most people had similar glycaemic control levels in childhood and adulthood, the contribution of metabolic memory (the concept that hyperglycaemia appears to have a chronic rather than an acute effect on the development of complications)4 from the paediatric period was integral to this risk.
Box 1 – Participant characteristics, HbA1c levels, and complication rates for all participants and for each glycaemic control trajectory group
|
|
All participants |
HbA1c trajectory group |
P* |
||||||||||||
|
Stable low |
Improving |
Worsening |
Stable high |
||||||||||||
|
|
|||||||||||||||
|
Number (% of participants) |
503 |
143 (28%) |
82 (16%) |
96 (19%) |
182 (36%) |
|
|||||||||
|
Sex (women) |
250 |
58 (40%) |
46 (56%) |
44 (46%) |
102 (56%) |
0.02 |
|||||||||
|
Age at diagnosis (years), mean (SD) |
9.9 (3.9) |
10.7 (4.1) |
9.1 (3.9) |
10.3 (4.2) |
9.5 (3.9) |
0.80 |
|||||||||
|
Duration of paediatric observation (years), mean (SD) |
8.5 (4.2) |
7.8 (4.1) |
9.2 (4.1) |
8.1 (4.6) |
8.9 (4.1) |
0.02 |
|||||||||
|
Range (years) |
0.6–19.1 |
1.0–19.1 |
3.8–19.1 |
1.1–18.3 |
0.6–18.9 |
|
|||||||||
|
Age at transition (years), mean (SD) |
18.4 (0.9) |
18.5 (0.8) |
18.3 (0.8) |
18.4 (1.1) |
18.4 (1.2) |
0.10 |
|||||||||
|
Duration of adult observation (years), mean (SD) |
8.9 (5.8) |
7.8 (5.3) |
10.0 (6.9) |
9.4 (5.5) |
9.2 (5.8) |
0.04 |
|||||||||
|
Range (years) |
0.3–21.8 |
0.8–20.9 |
0.4–21.8 |
0.3–20.9 |
0.5–21.6 |
|
|||||||||
|
Age at last follow-up (years), mean (SD) |
27.9 (6.3) |
26.4 (5.1) |
30.4 (7.7) |
27.6 (5.5) |
28.4 (6.3) |
< 0.001 |
|||||||||
|
Duration of type 1 diabetes, (years), mean (SD) |
18.1 (7.5) |
15.5 (6.7) |
21.3 (8.6) |
17.5 (7.4) |
18.9 (7.0) |
0.07 |
|||||||||
|
HbA1c measurements, mean number (SD) |
|||||||||||||||
|
Paediatric |
22.0 (13.0) |
21.9 (12.8) |
21.9 (15.5) |
19.9 (12.8) |
22.3 (12.8) |
0.50 |
|||||||||
|
Adult |
10.0 (8.1) |
11.3 (8.9) |
11.1 (9.5) |
9.5 (8.0) |
8.6 (8.1) |
0.40 |
|||||||||
|
Lifetime |
29.6 (15.9) |
33.3 (15.4) |
28.5 (18.0) |
27.9 (14.3) |
28.1 (15.8) |
0.17 |
|||||||||
|
HbA1c level (mmol/mol), mean (SD) |
|||||||||||||||
|
Paediatric |
68 (13.1) |
57 (6.6) |
74 (9.8) |
60 (5.5) |
78 (9.8) |
< 0.001 |
|||||||||
|
Adult |
70 (17.5) |
57 (6.6) |
60 (5.5) |
77 (10.9) |
85 (17.5) |
< 0.001 |
|||||||||
|
Lifetime |
68 (12.0) |
58 (3.3) |
67 (8.7) |
65 (6.6) |
79 (9.8) |
< 0.001 |
|||||||||
|
Severe complications |
26 (5.2%) |
1 (1%) |
6 (7%) |
3 (3%) |
16 (9%) |
0.006 |
|||||||||
|
Severe retinopathy |
16 (3.2%) |
0 |
3 (4%) |
1 (1%) |
12 (7%) |
0.004 |
|||||||||
|
Renal disease |
8 (2%) |
0 |
0 |
4 (5%) |
2 (2%) |
0.07 |
|||||||||
|
Ulceration/amputation |
4 (1%) |
1 (1%) |
1 (1%) |
0 |
4 (2%) |
0.76 |
|||||||||
|
Death |
5 (1%) |
0 |
0 |
1 (1%) |
4 (2%) |
0.18 |
|||||||||
|
|
|||||||||||||||
|
* Differences between trajectory groups tested in χ2 (categorical) and one-way ANOVA analyses (continuous variables). |
|||||||||||||||
Received 14 June 2016, accepted 30 November 2016
- Mary White1,2
- Matthew A Sabin1,3
- Costan G Magnussen4,5
- Michele A O'Connell1
- Peter G Colman1,6
- Fergus Cameron1
- 1 The Royal Children's Hospital, Melbourne, VIC
- 2 Monash Children's Hospital, Melbourne, VIC
- 3 University of Melbourne, Melbourne, VIC
- 4 Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS
- 5 Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland
- 6 Royal Melbourne Hospital, Melbourne, VIC
We acknowledge the assistance of the staff of BioGrid Australia during planning, ethics applications and data extraction for this article, particularly Leon Heffer and Knight Wang.
No relevant disclosures.
- 1. DCCT Writing Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977-986.
- 2. DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994; 125: 177-188.
- 3. Pambianco G, Costacou T, Ellis D, et al. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications study experience. Diabetes 2006; 55: 1463-1469.
- 4. DCCT Writing Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002; 287: 2563-2569.
- 5. Svensson M, Eriksson JW, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care 2004; 27: 955-962.
- 6. Miller RG, Secrest AM, Sharma RK, et al. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 2012; 61: 2987-2992.
- 7. Hirose A, Furushima D, Yamaguchi N, et al. Prediction of retinopathy at 20 years after onset in younger-onset type 1 diabetes using mean metabolic memory-free HbA1c values: the importance of using HbA1c data of total, not partial, diabetes duration. Diabetes Care 2013; 36: 3812-3814.
- 8. American Diabetes Association. Standards of medical care in diabetes — 2014. Diabetes Care 2014; 37 Suppl 1: S14-S80.
- 9. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 Suppl 1: S1-S266.
- 10. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604-612.
- 11. Rewers MJ, Pillay K, de Beaufort C, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2014; 15 Suppl 20: 102-114.
- 12. Australian Institute of Health and Welfare. Diabetes among young Australians (AIHW Cat. No. CVD 59; Diabetes Series No. 18). Canberra: AIHW, 2012.
- 13. White M, O’Connell MA, Cameron FJ. Transition in type 1 diabetes mellitus from a tertiary pediatric center: what are we doing before they walk out the door? Diabetes Manag 2012; 2: 379-384.
- 14. Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes 2016; 17: 327-336.
- 15. Cooper MN, de Klerk NH, Jones TW, et al. Clinical and demographic risk factors associated with mortality during early adulthood in a population-based cohort of childhood-onset type 1 diabetes. Diabet Med 2014; 31: 1550-1558.
- 16. Carlsen S, Skrivarhaug T, Thue G, et al. Glycemic control and complications in patients with type 1 diabetes: a registry-based longitudinal study of adolescents and young adults. Pediatr Diabetes 2016; doi: 10.1111/pedi.12372 [Epub ahead of print].





Abstract
Objectives: To determine the relationship between glycaemic control trajectory and the long term risk of severe complications in people with type 1 diabetes mellitus, as well as the effects of paediatric and adult HbA1c levels.
Design, setting, participants: Data linkage study of data for adults with childhood-onset type 1 diabetes (diagnosed during 1975–2010) who had transitioned from paediatric diabetes care at the Royal Children’s Hospital (Melbourne) to adult diabetes care at the Royal Melbourne Hospital during 1992–2013.
Main outcome measures: Severe complications were categorised as severe diabetic retinopathy (SDR), chronic kidney disease, ulceration or amputation, and death. Mean HbA1c levels were calculated for the paediatric and adult periods. Four glycaemic control trajectories were defined according to mean paediatric and adult HbA1c levels: stable low (paediatric and adult HbA1c ≤ 66 mmol/mol); improving (paediatric HbA1c > 66 mmol/mol, adult HbA1c ≤ 66 mmol/mol); worsening (paediatric HbA1c ≤ 66 mmol/mol, adult HbA1c > 66 mmol/mol); and stable high (paediatric and adult HbA1c > 66 mmol/mol).
Results: 503 eligible participants (253 men) were identified, 26 (5.2%) of whom had at least one severe complication, including 16 with SDR (3.2%). No-one in the stable low group, but 4% of the improving, 1% of the worsening, and 7% of the stable high groups developed SDR. Higher mean paediatric (per 10.9 mmol/mol increase: odds ratio [OR], 2.9; 95% CI, 1.9–4.3; P < 0.01) or adult HbA1c levels (OR, 2.1; 95% CI, 1.4–3.1; P < 0.01) were associated with increased risk of SDR, as was longer duration of type 1 diabetes (per additional year: OR, 1.3; 95% CI, 1.2–1.5; P < 0.01).
Conclusion: SDR was associated with higher paediatric HbA1c levels, independent of glycaemic control during adulthood; it was not documented in patients with a stable low glycaemic control trajectory.