Thyroid disease can be broadly categorised as thyroid dysfunction (hypothyroidism, hyperthyroidism) and structural disease (goitre, nodules and cancer). Management is often straightforward, but there are pitfalls that may lead to misdiagnosis, overdiagnosis and inappropriate treatment. This article reviews the approach to common thyroid problems in general practice.
Epidemiology
Worldwide, iodine deficiency is the most common cause of thyroid disease. Iodine deficiency has long been known in Tasmania, and a survey published in 2006 found evidence of iodine deficiency in mainland Australia, particularly New South Wales and Victoria.1 In 2009, use of iodised salt in bread became mandatory in Australia and New Zealand (following a Tasmanian program started in 2001), and Australia is now considered iodine sufficient.2
Autoimmune thyroid disease is the commonest cause of thyroid dysfunction in Australia, with prevalence as shown in Box 1. Some 10–15% of the population have positive thyroid antibodies, most commonly to thyroid peroxidase (TPOAb), with a higher prevalence in women than men.3 Patients who are euthyroid with positive thyroid antibodies do not require treatment, but are at increased risk of thyroid dysfunction (particularly hypothyroidism),4,5 and should be followed with annual measurement of serum thyroid-stimulating hormone (TSH).
TSH testing is the most sensitive means of detecting thyroid dysfunction. Debate surrounding the laboratory reference interval for TSH has largely resolved, and a range of about 0.4–4.0 mU/L is generally accepted.6,7 TSH concentrations increase with age,7,8 and some laboratories have adopted age-related reference intervals, with upper limits of up to 7 mU/L in older patients.
Hypothyroidism
Diagnosis
Classic symptoms of hypothyroidism include fatigue, weight gain, cold intolerance, arthralgia, constipation, menorrhagia, and dry skin and hair. Physical signs include pallor, coarse skin and hair, bradycardia and goitre, but may be absent in mild hypothyroidism. These symptoms and signs are non-specific and common in people without thyroid disease,9 so laboratory diagnosis is required. Serum TSH should be measured; if this is in the reference range, then additional tests such as free thyroxine (T4), free triiodothyronine (T3) or thyroid antibodies are rarely helpful. Tests such as basal metabolic rate and reverse free T3 have no diagnostic value.
Overt hypothyroidism (high TSH, low free T4) is usually symptomatic, readily diagnosed and can be treated without further investigation. A more common presentation in general practice is an elevated level of serum TSH with normal free T4. This may indicate subclinical hypothyroidism caused by autoimmune thyroid disease, but can arise from non-thyroidal, systemic illness, particularly in the recovery phase. In patients with a mildly elevated TSH (up to 10 mU/L), TSH normalises without treatment in over 50% of cases,10 so treatment need not be offered immediately. Instead, serum TSH testing should be repeated 6–8 weeks later, together with free T4 and TPOAb, and treatment offered if the abnormality persists. Thyroid imaging (including ultrasound) is not indicated in the investigation of hypothyroidism.11
The importance of detecting and treating subclinical hypothyroidism remains uncertain.12,13 Randomised controlled trials of thyroxine have shown inconsistent results on symptoms and quality of life, particularly in older patients and when TSH levels are mildly elevated (up to 10 mU/L). Untreated subclinical hypothyroidism with TSH levels above 10 mU/L is associated with increased risks of cardiovascular disease.14,15 The benefits of treating subclinical hypothyroidism are probably greater in younger and middle-aged patients than in older people.13,16
Treatment
Thyroid hormone replacement is indicated for overt hypothyroidism and for subclinical hypothyroidism with TSH levels above 10 mU/L. Patients with persistent mild subclinical hypothyroidism (TSH, 4–10 mU/L) and minimal or no symptoms can be offered a choice between thyroxine treatment or observation with annual follow-up testing to detect progressive hypothyroidism. Progression is more likely in TPOAb-positive patients.4 Women with subclinical hypothyroidism who are planning pregnancy should be treated (see below). When it is uncertain whether non-specific symptoms are caused by, or merely coexist with, mild subclinical hypothyroidism, a 3-month trial of thyroxine is reasonable to assess symptomatic benefit. Thyroid replacement therapy is not indicated for individuals with symptoms suggestive of hypothyroidism if TSH levels are within the reference interval.17
Thyroxine is the standard treatment for hypothyroidism.18 The usual approach is an initial dose of 50–100 μg/day with subsequent titration based on thyroid function tests checked 6–8 weeks later. Smaller initial doses (25 μg/day) should be used in very frail or elderly patients and in those with symptomatic ischaemic heart disease. Ideally, thyroxine should be taken in a fasting state, 1 hour before breakfast, but this may be inconvenient and reduce adherence, and it is probably more important that daily dosing is consistent with regard to time of day and relationship to meals.18 The long half-life of thyroxine means that if a dose has been missed, a catch-up dose can be taken later in the day or the following day.
When treating hypothyroidism, the targets are relief of symptoms and return of TSH to within the reference interval. In thyroxine-treated patients, serum free T4 may be within the reference range or elevated; the latter is not an indication to reduce dosage if TSH is within the reference interval. Measurement of free T3 is unhelpful in monitoring thyroxine replacement.11,18 In some patients, TSH levels remain elevated despite apparently adequate thyroxine dosage. The most common cause is non-adherence; other causes and ways to address these are shown in Box 2.
Three thyroxine preparations are available in Australia. Two of these (Eutroxsig and Oroxine, Aspen Pharma) are identical and interchangeable. A third preparation, Eltroxin (Aspen Pharma), has recently been marketed. It has a different formulation, a wider range of tablet strengths and (unlike Eutroxsig/Oroxine) does not require refrigeration, so may be more convenient. Eltroxin product information states that it is not bioequivalent to Oroxine/Eutroxsig, but this is based on a study using single, large doses of thyroxine in healthy volunteers, which may not predict clinically relevant differences during clinical use.18 Because of this uncertainty, patients should not be switched between Eltroxin and Eutroxsig/Oroxine (or vice versa), except where explicitly intended by the prescribing doctor. Patients who do switch brands should have their serum TSH checked 6 weeks later, and dosage adjusted if necessary.
Dissatisfaction with thyroxine replacement
In some patients, symptoms of ill health persist despite adherence to treatment and normalisation of TSH.18,19 There are three likely explanations for this. First, the persistent symptoms may be unrelated to thyroid dysfunction. For example, a patient may present with fatigue and be found to have mild subclinical hypothyroidism, but without a causal relationship between the two. In such cases, comorbidities including coeliac disease (which is associated with autoimmune thyroid disease) and depression should be sought to account for the symptoms, but often, none can be identified.
Second, standard thyroid replacement therapy may in some way be suboptimal for some patients. Anecdotally, some patients feel better if thyroxine dosage is increased until serum TSH levels are in the lower part of the reference interval (0.4–2.0 mU/L) or below the interval (0.1–0.4 mU/L), although this has not been confirmed in clinical trials.20 This approach appears to be safe, particularly in younger, otherwise healthy patients, so long as TSH is not suppressed below 0.1 mU/L.21 The healthy thyroid secretes small amounts of T3 as well as T4, and randomised controlled trials have explored whether the addition of T3 to thyroxine treatment results in symptomatic benefit. Overall, no convincing superiority of combined thyroxine/T3 treatment has been shown,18,22,23 but it remains possible that there is a subgroup of patients who respond symptomatically. Dessicated thyroid extract (from porcine thyroid) is sometimes prescribed for hypothyroidism. In a clinical trial, its effects on hypothyroid symptoms and neurocognitive function were equivalent to those of thyroxine, but resulted in modest weight loss compared with thyroxine (1.3 kg over 4 months) and was preferred by some participants.24 It is not approved by the Therapeutic Goods Administration or listed on the Pharmaceutical Benefits Scheme, and is not considered standard therapy.
Third, autoimmune thyroiditis is an inflammatory disorder associated with increased cytokine production, and it is possible that this causes symptoms of ill health, independent of thyroid dysfunction. In some studies, selenium supplementation (100–200 μg/day, equivalent to ingestion of two to four Brazil nuts per day) reduces inflammatory markers and improves quality of life in patients with Hashimoto’s disease.25 Although not an established treatment, this can be considered for patients with persistent symptoms.
Central (secondary) hypothyroidism
In patients with pituitary disease, serum TSH is unreliable in diagnosing central hypothyroidism and in monitoring thyroxine replacement. Monitoring is based on free T4 measurements and clinical assessment,18 and should include specialist input.
Hyperthyroidism
Overt hyperthyroidism
Hyperthyroidism is less common than hypothyroidism. The clinical picture is often characteristic, with symptoms including weight loss, heat intolerance, palpitations, breathlessness, anxiety, diarrhoea, tremor and proximal muscle weakness. Physical signs include tremor, tachycardia, ophthalmopathy, goitre and difficulty rising from a squatting position. The diagnosis is confirmed by thyroid function tests showing suppressed TSH (usually undetectable) with elevated free T4 and/or free T3.26
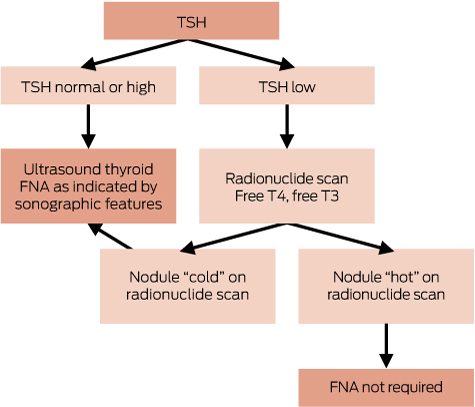
Hyperthyroidism is most commonly caused by Graves’ disease, thyroiditis or toxic nodular goitre. It is important to establish the cause of hyperthyroidism before starting treatment: Box 3 shows the key clinical features and diagnostic tests. Positive TSH-receptor antibodies (TRAb) establish a diagnosis of Graves’ disease. Radionuclide thyroid scanning is often helpful (Box 4). Thyroid ultrasound is not routinely indicated; it does not distinguish reliably between Graves’ disease and thyroiditis, and frequently identifies nodules that are unrelated to the hyperthyroidism, resulting in diagnostic confusion and further unnecessary investigation.11,26
Thyroiditis characteristically has a triphasic course of hyperthyroidism followed by hypothyroidism, resolving to euthyroidism. In subacute (viral) thyroiditis, full recovery is the rule, whereas in autoimmune thyroiditis (with positive TPOAb), hypothyroidism may persist. Often no treatment is required during the thyrotoxic phase, although β-blockers may be helpful symptomatically. TSH, free T4 and free T3 should be checked every 6–8 weeks until resolution. If hypothyroidism persists, then thyroxine treatment is indicated.
Graves’ disease should be treated initially with carbimazole (15–20 mg daily for mild to moderate hyperthyroidism, 30–40 mg for severe hyperthyroidism). In patients who respond well, it can be continued for an 18-month course, aiming for long term remission. Other treatment options are radioactive iodine treatment and thyroidectomy.
Toxic nodular goitre can be treated with surgery or radioactive iodine. Antithyroid drugs can be used, but need to be continued lifelong as remission of hyperthyroidism is unlikely, and are not the preferred option.
Hyperthyroid patients with Graves’ disease, toxic nodular goitre and those in whom the diagnosis is unclear should generally be referred.
Subclinical hyperthyroidism
Mild subclinical hyperthyroidism, with TSH levels between 0.1 and 0.4 mU/L, may be caused by autonomous thyroid nodules but may also be found in healthy individuals (healthy outliers). It often resolves without treatment,10 so follow-up with repeat testing may be all that is required. Subclinical hyperthyroidism with TSH levels persistently below 0.1 mU/L is classified as mild hyperthyroidism and should be managed as above.
Thyroid nodules and cancer
Palpable thyroid nodules
Palpable thyroid nodules are present in about 5% of the population.27,28 Most are benign, commonly colloid nodules, cysts, nodular thyroiditis or benign neoplasm, whereas about 5% are malignant. Large nodular goitres can be symptomatic and require surgery for relief of pressure symptoms, but most thyroid nodules are asymptomatic, and the diagnostic work-up is aimed at assessing the risk of thyroid cancer. The diagnostic approach to palpable thyroid nodules is shown in Box 5. TSH levels should be measured but are usually normal, and the key investigation is ultrasound-guided fine needle aspiration (FNA) biopsy. Where clinical assessment, sonographic features and cytology are all consistent with a benign pathology, no further assessment is required. When cytology is suspicious of cancer or indeterminate, or if clinical suspicion persists, referral to an endocrine surgeon or head and neck surgeon is indicated. If there is uncertainty regarding the need for surgery, an opinion from an endocrinologist may be helpful.
When TSH is suppressed, radionuclide scanning may detect one or more autonomous (“hot”) nodules. These are rarely malignant, and do not routinely require biopsy. When TSH is normal or raised, radionuclide scanning is not indicated.
Thyroid nodules and cancer: the risk of overdiagnosis
Although 5% of people have a palpable thyroid nodule, the prevalence of nodules detectable by ultrasound is much higher, and increases with age up to 70% in the elderly.27 Clinically diagnosed thyroid cancer is uncommon, with a lifetime risk of less than 1%. In autopsy studies, however, small thyroid cancers are present in up to 36% of individuals.29 Most of these are small papillary cancers < 1 cm in size.
In Australia and many other countries, the incidence of thyroid cancer is increasing dramatically.30-32 The increase is largely accounted for by small papillary cancers found using ultrasound, most of which would never have become clinically apparent, an example of overdiagnosis.33 The most extreme example is in Korea, where screening with thyroid ultrasound has resulted in a 15-fold increase in thyroid cancer incidence, with no reduction in mortality (which is very low).34
Australia can avoid an epidemic of thyroid cancer by judicious use of thyroid ultrasound as follows:
Thyroid ultrasound should be performed to assess clinically detected, visible or palpable thyroid nodules or goitre. It is not indicated for hypothyroidism or hyperthyroidism in the absence of goitre; nor is it indicated for globus, non-specific symptoms, or for screening.
FNA biopsy should be considered for nodules > 1 cm in size, based on sonographic appearances. Nodules < 1 cm in size should not be routinely biopsied.27,33
Thyroid disease in pregnancy and postpartum
Pregnancy requires a 30–50% increase in thyroid hormone secretion because of stimulatory effects of chorionic gonadotropin (hCG) on the thyroid, increased circulating levels of thyroxine-binding globulin and degradation of thyroid hormone by the placenta.35 Maternal T4 crosses the placenta and is important for fetal brain development until 18–20 weeks’ gestation, when the fetal thyroid is fully functional. Mild iodine deficiency during pregnancy may impair fetal brain development,36 and dietary sources of iodine may not be sufficient for increased requirements during pregnancy.37 Iodine supplementation (150 μg/day) is therefore recommended for women who are pregnant or trying to conceive.
It remains controversial whether universal screening of pregnant women for thyroid dysfunction is indicated.38,39 The main value of screening is probably the detection of rare cases of overt hypothyroidism and hyperthyroidism for which treatment is clearly indicated, rather than minor abnormalities of uncertain significance (which are more common).35 Where screening is performed, TSH should be measured during the first trimester.
General laboratory reference intervals for TSH and free T4 do not apply to pregnancy. American Thyroid Association guidelines recommend that laboratories should develop trimester- and method-specific reference ranges from local populations. Instead, many laboratories have simply adopted suggested TSH reference intervals from the guidelines as follows: first trimester, 0.1–2.5 mU/L; second trimester, 0.2–3.0 mU/L; and third trimester, 0.3–3.0 mU/L.38 These intervals were originally based on expert opinion and limited data. Subsequent reference interval studies from different countries have produced widely varying results; in most, the upper limit for TSH is higher than 2.5 or 3.0 mU/L,40-43 suggesting that these cut-offs may not be appropriate.
Hypothyroidism
Overt hypothyroidism during pregnancy is associated with adverse outcomes, including miscarriage, pre-eclampsia, placental abruption, preterm birth, low birth weight and reduced IQ in offspring.38,39 The impact of subclinical hypothyroidism during pregnancy is uncertain. Although some observational studies have shown adverse outcomes,44 the data are inconsistent and some studies have found no association.45,46
There are few clinical trials of thyroxine in pregnancy. In one study, thyroxine treatment of TPOAb-positive, euthyroid pregnant women resulted in fewer miscarriages and preterm births.47 More recently, thyroxine treatment of pregnant women with elevated TSH or reduced free T4 concentrations at a mean gestation of 12 weeks had no effect on obstetric outcomes or on cognitive function in the offspring.48 Other trials are in progress.
Until better data are available, the following approach is recommended. Women with pre-existing hypothyroidism who are planning a pregnancy should have their thyroxine dose optimised, aiming for serum TSH in the lower reference range (0.4–2.5 mU/L). When pregnancy is confirmed, thyroxine dosage should be increased by about 30%, conveniently achieved by doubling the thyroxine dose on 2 days per week (with no change on other days). Serum TSH should be checked every 4–6 weeks until 20 weeks’ gestation, then once in the third trimester. After delivery, thyroxine dosage can be reduced to pre-pregnancy levels and TSH levels checked at 6–8 weeks postpartum.38,39
In women found to have elevated serum TSH during pregnancy, free T4 and TPOAb should be measured. If TSH is > 4 mU/L or free T4 is reduced, thyroxine treatment should be started, at an initial dose of 75–100 μg/day.40
It is uncertain whether pregnant women with TSH values in the 2.5–4.0 mU/L range benefit from thyroxine treatment. American Endocrine Society guidelines recommend thyroxine treatment for all such women,39 but this is increasingly challenged as overly simplistic, and is likely to result in overdiagnosis of subclinical hypothyroidism and unnecessary treatment.41-43,49 American Thyroid Association guidelines recommend treating pregnant women with TSH levels > 2.5 mU/L if they are TPOAb-positive.38
Hyperthyroidism in pregnancy
Overt hyperthyroidism during pregnancy is uncommon. Gestational hyperthyroidism can occur in the first trimester because of the stimulatory effect of hCG on the thyroid, mediated by the TSH receptor in women with very high hCG levels, particularly in hyperemesis gravidarum or multiple pregnancy. It usually resolves rapidly without treatment. Persistent hyperthyroidism in pregnancy is usually caused by Graves’ disease. Radionuclide scanning is contraindicated in pregnancy, and the key diagnostic test is measurement of TRAb. Hyperthyroidism increases the risk of pregnancy loss and other adverse outcomes, and patients should be referred urgently.
Subclinical hyperthyroidism can be a normal variant in pregnancy, or can be caused by thyroid disease. It is not associated with adverse outcomes and may not require treatment,50 but should be monitored closely.
Postpartum thyroid dysfunction
Thyroid dysfunction occurs in up to 10% of women in the first year postpartum. Hyperthyroidism may be caused by postpartum (autoimmune) thyroiditis or Graves’ disease, which can be distinguished by measurement of serum TRAb or radionuclide scanning (although scanning is contraindicated in breastfeeding women). Postpartum thyroiditis can be managed as above; it results in long term hypothyroidism in 10–20% of affected women.38,39
Box 1 – Prevalence of thyroid disease in Australia
Condition |
Definition |
Prevalence |
|||||||||||||
Thyroid autoimmunity |
Positive TPOAb or TgAb |
12% |
|||||||||||||
Subclinical hypothyroidism |
Increased TSH, normal free T4 |
5% |
|||||||||||||
Overt hypothyroidism |
Increased TSH, low free T4 |
0.5% |
|||||||||||||
Subclinical hyperthyroidism |
Decreased TSH, normal free T4 and free T3 |
0.3% |
|||||||||||||
Overt hyperthyroidism |
Decreased TSH, elevated free T4 and/or free T3 |
0.3% |
|||||||||||||
Palpable thyroid nodules |
About 5% |
||||||||||||||
Thyroid nodules at ultrasound |
Increases with age, up to 70% of the elderly |
||||||||||||||
T3 = triiodothyronine. T4 = thyroxine. TgAb = thyroglobulin antibodies. TPOAb = thyroid peroxidase antibodies. TSH = thyroid-stimulating hormone. | |||||||||||||||
Box 2 – Causes of persistent elevation of thyroid-stimulating hormone levels despite thyroxine replacement
Cause |
Remedies |
||||||||||||||
Inadequate dosage |
Increase dose |
||||||||||||||
Non-adherence |
Monitor and encourage adherence (eg, Webster pack)Supervised dosing (including weekly administration of a full week’s dose) |
||||||||||||||
Thyroxine expired, heat- or moisture-exposed |
Replace tablets |
||||||||||||||
Thyroxine absorption reduced by food |
Take thyroxine with water in fasting state, 60 minutes before breakfast; or at bedtime, 3 hours after evening meal |
||||||||||||||
Co-administration with other medications (calcium carbonate, ferrous sulphate, multivitamins, sucralfate, raloxifene) |
Separate timing of thyroxine from other medications (eg, thyroxine in morning and other medications at night) |
||||||||||||||
Malabsorption from bowel disease |
Investigate for bowel disease (coeliac antibodies, upper gastrointestinal endoscopy) |
||||||||||||||
Box 3 – Common causes of hyperthyroidism and their management
Disease |
Other names |
Pathogenesis |
Clinical pointers |
Key investigations |
Initial treatment |
||||||||||
Graves’ disease |
Stimulating antibodies to TSH receptor, causing increased thyroid hormone secretion |
Diffuse goitreThyroid bruitOphthalmopathy |
Radionuclide scanTSH-receptor antibodies |
Antithyroid drugs |
|||||||||||
Lymphocytic thyroiditis |
Hashitoxicosis, silent thyroiditis, painless thyroiditis; includes postpartum thyroiditis |
Destructive, autoimmune thyroiditis causing release of stored thyroid hormoneTransient hyperthyroidism, may be followed by transient or permanent hypothyroidism |
Often none |
Radionuclide scanThyroid peroxidase antibodies |
Usually none (consider β-blockers if symptomatic) |
||||||||||
Subacute thyroiditis |
De Quervain thyroiditis, viral thyroiditis |
Destructive, viral thyroiditis causing release of stored thyroid hormoneTransient hyperthyroidism, may be followed by transient hypothyroidism |
Preceding viral illnessPainful, tender thyroid |
Radionuclide scanC-reactive protein |
Usually none (consider β-blockers if symptomatic)For painful thyroiditis: analgesia, NSAIDs; rarely prednisolone |
||||||||||
Toxic nodular goitre |
Toxic adenoma |
Single or multiple adenomas secreting thyroid hormone |
Asymmetric, irregular goitre |
Radionuclide scan |
None or antithyroid drugs |
||||||||||
NSAIDs = non-steroidal anti-inflammatory drugs. TSH = thyroid-stimulating hormone. | |||||||||||||||
Box 4 – Radionuclide scanning appearances of the thyroid in hyperthyroidism

A: Graves’ disease, with diffusely increased tracer uptake. B: Thyroiditis, with absent thyroidal uptake of tracer. C: Solitary autonomous nodule with focal tracer uptake in left lobe and reduced uptake in right lobe. D: Toxic multinodular goitre, with multiple areas of increased and reduced uptake.
Provenance: <p>Commissioned; externally peer reviewed.</p>
- John P Walsh1,2
- 1 Sir Charles Gairdner Hospital, Perth, WA
- 2 University of Western Australia, Perth, WA
No relevant disclosures.
- 1. Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust 2006; 184: 165-169. <MJA full text>
- 2. Australian Bureau of Statistics. Australian Health Survey: Biomedical Results for Nutrients, 2011-12. Feature Article: Iodine. Canberra: ABS, 2013. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.006Chapter1202011-12 (accessed June 2016).
- 3. O’Leary PC, Feddema PH, Michelangeli VP, et al. Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. Clin Endocrinol (Oxf) 2006; 64: 97-104.
- 4. Walsh JP, Bremner AP, Feddema P, et al. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab 2010; 95: 1095-1104.
- 5. Asvold BO, Vatten LJ, Midthjell K, Bjoro T. Serum TSH within the reference range as a predictor of future hypothyroidism and hyperthyroidism: 11-year follow-up of the HUNT Study in Norway. J Clin Endocrinol Metab 2012; 97: 93-99.
- 6. Brabant G, Beck-Peccoz P, Jarzab B, et al. Is there a need to redefine the upper normal limit of TSH? Eur J Endocrinol 2006; 154: 633-637.
- 7. Laurberg P, Andersen S, Carle A, et al. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7: 232-239.
- 8. Kahapola-Arachchige KM, Hadlow N, Wardrop R, et al. Age-specific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clin Endocrinol (Oxf) 2012; 77: 773-779.
- 9. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med 2000; 160: 526-534.
- 10. Meyerovitch J, Rotman-Pikielny P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med 2007; 167: 1533-1538.
- 11. Endocrine Society of Australia. Top 5 low-value practices and interventions: Sydney: Royal Australasian College of Physicians, 2015. http://evolve.edu.au/published-lists/esa (accessed Apr 2016).
- 12. Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US Preventive Services Task Force. Ann Intern Med 2015; 162: 35-45.
- 13. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29: 76-131.
- 14. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304: 1365-1374.
- 15. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012; 126: 1040-1049.
- 16. Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172: 811-817.
- 17. Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: randomised double blind placebo controlled crossover trial. BMJ 2001; 323: 891-895.
- 18. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid 2014; 24: 1670-1751.
- 19. Walsh JP. Dissatisfaction with thyroxine therapy - could the patients be right? Curr Opin Pharmacol 2002; 2: 717-722.
- 20. Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91: 2624-2630.
- 21. Flynn RW, Bonellie SR, Jung RT, et al. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 2010; 95: 186-193.
- 22. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 2003; 88: 4543-4550.
- 23. Grozinsky-Glasberg S, Fraser A, Nahshoni E, et al. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2006; 91: 2592-2599.
- 24. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab 2013; 98: 1982-1990.
- 25. van Zuuren EJ, Albusta AY, Fedorowicz Z, et al. Selenium supplementation for Hashimoto’s thyroiditis. Cochrane Database Syst Rev 2013; 6: CD010223.
- 26. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 2011; 21: 593-646.
- 27. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26: 1-133.
- 28. Hegedüs L, Bonnema SJ, Bennedbæk FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev 2003; 24: 102-132.
- 29. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer 1985; 56: 531-538.
- 30. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014; 140: 317-322.
- 31. Pandeya N, McLeod DS, Balasubramaniam K, et al. Increasing thyroid cancer incidence in Queensland, Australia 1982-2008-true increase or overdiagnosis? Clin Endocrinol (Oxf) 2016; 84: 257-264.
- 32. Burgess JR. Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982-1997). Thyroid 2002; 12: 141-149.
- 33. Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ 2013; 347: f4706.
- 34. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”–screening and overdiagnosis. N Engl J Med 2014; 371: 1765-1767.
- 35. Laurberg P, Andersen SL, Pedersen IB, et al. Screening for overt thyroid disease in early pregnancy may be preferable to searching for small aberrations in thyroid function tests. Clin Endocrinol (Oxf) 2013; 79: 297-304.
- 36. Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013; 98: 1954-1962.
- 37. Burgess JR, Seal JA, Stilwell GM, et al. A case for universal salt iodisation to correct iodine deficiency in pregnancy: another salutary lesson from Tasmania. MJA 2007; 186: 574-576.
- 38. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21: 1081-1125.
- 39. De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97: 2543-2565.
- 40. Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349: g4929.
- 41. Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351: h4726.
- 42. Medici M, Korevaar TI, Visser WE, et al. Thyroid function in pregnancy: what is normal? Clin Chem 2015; 61: 704-713.
- 43. Laurberg P, Andersen SL, Hindersson P, et al. Dynamics and predictors of serum TSH and fT4 reference limits in early pregnancy. A study within the Danish National Birth Cohort. J Clin Endocrinol Metab 2016: 101: 2484-2492.
- 44. Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26: 580-590.
- 45. Ong GS, Hadlow NC, Brown SJ, et al. Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation? J Clin Endocrinol Metab 2014; 99: E2668-E2672.
- 46. Mannisto T, Vaarasmaki M, Pouta A, et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab 2009; 94: 772-779.
- 47. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91: 2587-2591.
- 48. Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med 2012; 366: 493-501.
- 49. Brabant G, Peeters RP, Chan SY, et al. Management of subclinical hypothyroidism in pregnancy: are we too simplistic? Eur J Endocrinol 2015; 173: P1-P11.
- 50. Casey BM, Dashe JS, Wells CE, et al. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol 2006; 107: 337-341.





Summary