Abstract
Objective: To assess whether Australian clinical trials activity in National Health Priority Areas (NHPAs) reflects the relative disease burden.
Design and setting: Analysis of trials registered on the Australian New Zealand Clinical Trials Registry (ANZCTR) or ClinicalTrials.gov from January 2008 to December 2012 that planned recruitment in Australia and investigated interventions for NHPA conditions (cancer control, cardiovascular health, mental health, obesity, injury prevention/control, diabetes mellitus, arthritis and musculoskeletal conditions, dementia and asthma). Australian estimates of disability-adjusted life-years (DALYs) were used to quantify the burden of disease for each NHPA.
Main outcome measures: For each NHPA, the total number of registered trials, planned recruitment, and the predicted numbers based on disability-adjusted life-years expressed as a proportion of the total burden of disease in Australia (%DALY).
Results: 5143 trials with Australian sites were registered in the 5-year study period with total planned recruitment of 2 404 609 participants. Of these, 3032 trials (59%) with planned recruitment of 1 532 064 participants (64%) investigated NHPA conditions. Trial numbers and planned recruitment were highest for cancer, cardiovascular and mental health — reflecting their higher disease burden. In contrast, planned recruitment into obesity and dementia trials was ≤ 50% of that predicted from total trial activity based on their relative disease burden. The number of registered trials for these conditions was also lower than predicted. Overall, of 3032 NHPA trials, 2335 (77%) used randomisation and 1520 (50%) planned to recruit > 100 participants.
Conclusions: Australian clinical trial activity for obesity and dementia interventions is lower that would be expected based on their relative disease burden. Trial registries provide a valuable public database to identify and monitor gaps in research activity.
To improve Australia's health, clinical research programs should devote substantial activity to advancing practice in areas of high clinical need. Clinical trials are designed to provide high-quality evidence of the effectiveness of new interventions to establish best clinical practice. However, few studies have examined the extent to which Australian clinical trials address priority areas of clinical need.
The Australian Institute of Health and Welfare (AIHW) National Health Priority Areas (NHPAs) were introduced to encourage appropriate targeting of health services and clinical research to improve health. Currently, there are nine NHPAs: cancer control, cardiovascular health, mental health, injury prevention and control, diabetes mellitus, obesity, arthritis and musculoskeletal conditions, dementia and asthma. These NHPAs account for approximately three-quarters of the total estimated burden of disease in Australia (1 915 600 of 2 632 800 disability-adjusted life-years [DALYs]).1
Previous studies have reported a disparity between the level of National Health and Medical Research Council (NHMRC) grant funding for studies investigating NHPA conditions relative to their disease burden.2,3 The founding of clinical trial registries, including the Australian New Zealand Clinical Trial Registry (ANZCTR) in 2005, provides the first opportunity to examine how well clinical trial activity in Australia is targeted to NHPAs.
Methods
We conducted a retrospective analysis using ANZCTR and ClinicalTrials.gov (CT.gov) data to report on Australian trial activity and characteristics for NHPAs; and to compare the level of trial activity to the relative burden of disease for each NHPA.
Ethics approval was not required for this analysis of publicly available trial data.
Data sources
Trial registration is voluntary in Australia.4
The ANZCTR is an online public registry of clinical trials maintained by the NHMRC Clinical Trials Centre, the University of Sydney. It collects information about trial interventions, investigated health conditions, planned recruitment, outcomes, funding and sponsorship using the World Health Organization-defined 20-item minimum dataset.5 Health conditions are coded using the United Kingdom Clinical Research Collaboration Health Research Classification System (http://www.hrcsonline.net). Additional data are collected about trial design, including randomisation and blinding. The ANZCTR 2011 Data Quality and Completeness Audit reported that, on average, at least 93 of 94 data fields for 148 trials were complete.6
CT.gov is an online public registry of clinical trials maintained by the United States National Library of Medicine (https://clinicaltrials.gov). It records similar data items to the ANZCTR.
Trial sample and characteristics
The trial sample included all trials of health-related interventions registered on the ANZCTR or CT.gov between 1 January 2008 and 31 December 2012 that included Australia as a country of recruitment. To avoid entering duplicate trial data, trials that listed a CT.gov or ANZCTR registration number as a secondary identifier were only included in the ANZCTR trial list.
Condition categories and codes were used to classify individual trials as addressing one or more NHPA conditions, or other, non-NHPA conditions. For each trial, we extracted information for: purpose of intervention (treatment, prevention, diagnosis, education/counselling/training, other/missing); allocation of intervention (randomised, non-randomised); trial phase (I–IV, not applicable, missing), blinding (blinded, open, other/missing), planned recruitment (reported as target sample size, and classified as < 100, 100–1000, > 1000 participants); participant age range (< 18 years, 18–69 years, ≥ 70 years); and countries of recruitment (Australia only, Australia and overseas).
Analysis
To measure trial activity, we recorded the total number and planned recruitment of registered trials investigating NHPA conditions. To assess whether trial activity reflected the burden of disease for each NHPA, we compared the relative trial activity targeted to each NHPA, measured as a proportion of the total trial activity, with the “expected” distribution of trial activity estimated from the relative burden of disease for that NHPA. Burden of disease was estimated from published estimates of DALYs for each NHPA expressed as a percentage of the total burden of disease and injury in Australia (%DALY).1
To describe disparities in relative trial activity by NHPA, we identified NHPAs where the observed trial activity was less than 50% or more than 200% of expected values. The χ2 goodness-of-fit test was also used to test for statistically significant differences between observed and expected trial activity for each NHPA. For these analyses, a two-sided P < 0.006 was regarded as statistically significant using the Bonferroni adjustment for multiple comparisons (nine comparisons).
For assessment of trial recruitment across NHPA, we also conducted a sensitivity analysis to examine trial recruitment to NHPA from Australian sites, where Australian recruitment was estimated from the planned recruitment from all ANZCTR trials plus 10% of the planned recruitment from CT.gov trials that included at least one Australian site. The figure of 10% was estimated from a randomly selected sample of 100 CT.gov registered trials that included at least one Australian site and represents the number of Australian sites as a proportion of all sites for each trial.
We also calculated the frequency distribution of trial characteristics for each NHPA. SAS, version 9.3 (SAS Institute) was used for data analyses.
Results
There were 5143 intervention trials registered during 2008–2012 that planned to recruit in Australia (ANZCTR, 3379; CT.gov, 1764). Of these, 3032 (59%) related to NHPA conditions (ANZCTR, 1908; CT.gov, 1124). Total planned recruitment for the trial sample was 2 404 609 participants, including 1 532 064 (64%) for NHPA trials (ANZCTR, 670 832; CT.gov, 861 232).
Trial activity in NHPA
The three disease areas that contribute the largest %DALY — cancer, cardiovascular diseases and mental disorders — also attracted the largest number of trial registrations and the largest planned recruitment (Box 1; Box 2).
The proportions of registered trials that investigated dementia or injury interventions were less than half those expected from their %DALYs (65/185 [35%] and 137/360 [38%], respectively; Box 1). The proportions of obesity and asthma trials were also lower than expected (195/386 [51%] and 68/123 [55%], respectively). In contrast, the proportion of registered arthritis and musculoskeletal diseases trials was about twice as high as expected on the basis of the %DALY (Box 1).
The proportions of planned recruitment to trials investigating obesity and dementia were also substantially lower than expected from their %DALYs (33 948/180 346 [19%] and 24 248/86 566 [28%], respectively), and was also low for asthma (29 468/57 711 [51%]) (Box 1).
When this analysis was repeated using estimated recruitment from Australian sites only, a similar pattern was observed, with the exception of recruitment to diabetes trials. For diabetes trials, total trial planned recruitment was relatively high (185 929/132 253 [141%]) compared with Australian sites (44 201/66 607 [66%]).
Trial characteristics
Overall, 2335 of 3032 (77%) NHPA trials used a randomised design and 1509 (50%) planned recruitment of ≤ 100 participants (Box 3). Of the 2931 NHPA trials that reported information about blinding, 1504 (51%) reported using it (Box 3).
About three-quarters of NHPA intervention trials investigated treatments (2321 [76%]) and 397 (13%) investigated prevention interventions (Box 3). The ratio of treatment to prevention trials ranged from less than 2 : 1 for obesity trials to 14 : 1 for cancer trials.
Most NHPA trials excluded children, whereas 2252 (75%) specified a maximum participant age of ≥ 70 years, or did not specify a maximum age (Box 3). International recruitment sites were reported in 1081 (36%) of NHPA trials (169 ANZCTR trials, 912 CT.gov trials) and varied by condition (Box 3).
Discussion
This study provides the first overview of clinical trial activity in Australia. We found that more than half of Australian registered intervention trials and planned trial recruitment are targeted to NHPA conditions.
Trial activity for cancer, cardiovascular diseases and mental disorders was high relative to other NHPA conditions, consistent with their position as the three major contributors to disability and premature death in Australia. In contrast, trial activity for obesity and dementia interventions was substantially less than the level expected from their contribution to the total DALY.
To interpret these results, the number of trials can be considered to provide a proxy measure for the number of active research questions being investigated to identify more effective interventions in each area. Planned trial recruitment provides a measure of the number of patients actively participating in research to determine best practice in each area.
These findings suggest there is a need to further examine research activity for obesity, dementia and asthma to determine if and how clinical trials research in these areas should be increased. However, this study does not allow us to define the optimum level of trial activity for each condition. Clearly, not all important research questions for NHPAs are amenable to investigation through clinical trials. For conditions where trial activity is already high relative to other disease areas, further increases may still represent good value for money by improving health care. For example, if promising new interventions are available; or practice variations or controversies exist with gaps in evidence to guide best practice. Conversely, for some conditions where trial activity is currently low, research priorities may warrant other study designs, such as those used in translational research or behavioural science, to develop new interventions.
This study also provides the first opportunity to assess the extent to which Australian trials are designed to provide robust, high-quality evidence for guiding practice. The use of randomisation and blinding provides a measure of trial quality; trial size provides an indicator of study power. Trials enrolling more than 100 participants are generally required to assess clinically meaningful health outcomes and to weigh up the benefits and harms of the new strategy, whereas smaller trials are generally designed to assess surrogate outcomes. About three-quarters of Australian trials used a randomised design; however, only around half reported blinding, or planned recruitment of more than 100 participants. These findings are slightly more favourable than those of a recent analysis of 79 413 intervention trials registered on CT.gov between 2000 and 2010, which reported that 70% used a randomised design, 44% used a blinded design and 38% enrolled 100 or more participants.7
One commonly raised concern about clinical trials research is the applicability of trial data to routine clinical practice populations and settings. Our finding that more than two-thirds of trials in NHPA areas did not exclude participants aged 70 years or older is encouraging.
The main strength of our study is that it provides a unique, timely overview of Australian clinical trials to inform current debate on the achievements, limitations and future directions for clinical trials research in Australia. Clinical researchers can use the same methods to further explore gaps for conditions within specific disease areas, as has been performed for cancer trials.8
There are two main limitations to our study that could affect our estimates of trial activity in different directions. First, we relied on trial registrations to estimate trial activity. As trial registration is not compulsory in Australia, we may have underestimated trial activity. Additionally, we only included international trials registered on the ANZCTR or CT.gov. A search using the WHO International Clinical Trials Registry Platform Search Portal (http://www.who.int/ictrp/search/en) showed that 11 096 of 11 412 (97%) trials with Australian sites are registered on these two registries. The total number of registered trials may therefore be 3% higher than our study estimate.
Second, our estimates of trial participation may overestimate the number of Australians participating in clinical trials, because 1622 of 5143 trials (32%) included sites outside Australia. Nevertheless, by including Australian sites, these trial recruitment figures capture participation in trials that can be expected to provide evidence relevant to Australian practice.
Despite these limitations, we believe our findings are valuable in informing initiatives to increase clinical trial activity.9,10 It is well documented that trial research is often not available to guide many routine clinical decisions about selecting interventions.11 To guide practice, large trials with adequate long-term follow-up are needed to identify small incremental improvements in health outcomes and/or adverse events. Our findings on trial size suggest that further efforts are needed to promote and support the conduct of large trials, or support the conduct of small high-quality trials that can later contribute data to meta-analyses.
Overall, we demonstrate the feasibility and value of using publicly available trial registry data to examine the profile of trials research for particular conditions and identify gaps in trial activity to inform trial initiatives. The ANZCTR provides a valuable resource for researchers to ensure new studies build on, or contribute to, existing trials.
1 Number of registered Australian intervention trials and total planned recruitment in National Health Priority Areas, as a percentage of total trial activity, and comparison to the expected number based on %DALY, Australian New Zealand Clinical Trials Registry and ClinicalTrials.gov, 2008–2012
DALY | Trials | Planned recruitment | |||||||||||||
National Health Priority Area | Rank | % | Rank | Observed | Expected no. | Observed/ | P* | Rank | Observed no. (%) | Expected no. | Observed/ | P* | |||
Cancer control | 1 | 19.0% | 1 | 871 (16.9%) | 977 | 89% | 0.007 | 2 | 427 188 (17.8%) | 456 876 | 94% | < 0.001 | |||
Cardiovascular health | 2 | 18.0% | 3 | 646 (12.6%) | 926 | 70% | < 0.001 | 1 | 577 178 (24.0%) | 432 830 | 133% | < 0.001 | |||
Mental health | 3 | 13.3% | 2 | 693 (13.5%) | 684 | 101% | 0.82 | 3 | 196 826 (8.2%) | 319 813 | 62% | < 0.001 | |||
Obesity | 4 | 7.5% | 6 | 195 (3.8%) | 386 | 51% | < 0.001 | 7 | 33 948 (1.4%) | 180 346 | 19% | < 0.001 | |||
Injury prevention and control | 5 | 7.0% | 7 | 137 (2.7%) | 360 | 38% | < 0.001 | 5 | 125 256 (5.2%) | 168 323 | 74% | < 0.001 | |||
Diabetes mellitus | 6 | 5.5% | 5 | 282 (5.5%) | 283 | 100% | 1.00 | 4 | 185 929 (7.7%) | 132 253 | 141% | < 0.001 | |||
Arthritis and musculoskeletal conditions | 7 | 4.0% | 4 | 410 (8.0%) | 206 | 199% | < 0.001 | 6 | 109 107 (4.5%) | 96 184 | 113% | < 0.001 | |||
Dementia | 8 | 3.6% | 9 | 65 (1.3%) | 185 | 35% | < 0.001 | 9 | 24 248 (1.0%) | 86 566 | 28% | < 0.001 | |||
Asthma | 9 | 2.4% | 8 | 68 (1.3%) | 123 | 55% | < 0.001 | 8 | 29 468 (1.2%) | 57 711 | 51% | < 0.001 | |||
DALY = disability-adjusted life-years. %DALY = DALYs expressed as a proportion of the total burden of disease in Australia.1 Observed number of trials is expressed as a percentage of total 5143 registered intervention trials. Observed planned recruitment is expressed as a % of total 2 404 609 planned recruitment. Expected number of trials is calculated by applying %DALY to total 5143 registered intervention trials. Expected planned recruitment is calculated by applying %DALY to total 2 404 609 planned recruitment. * χ2 goodness-of-fit test for comparison of observed versus expected values.
2 Relationship between trial characteristics and %DALY for each NHPA, Australian New Zealand Clinical Trials Registry and ClinicalTrials.gov, 2008–2012
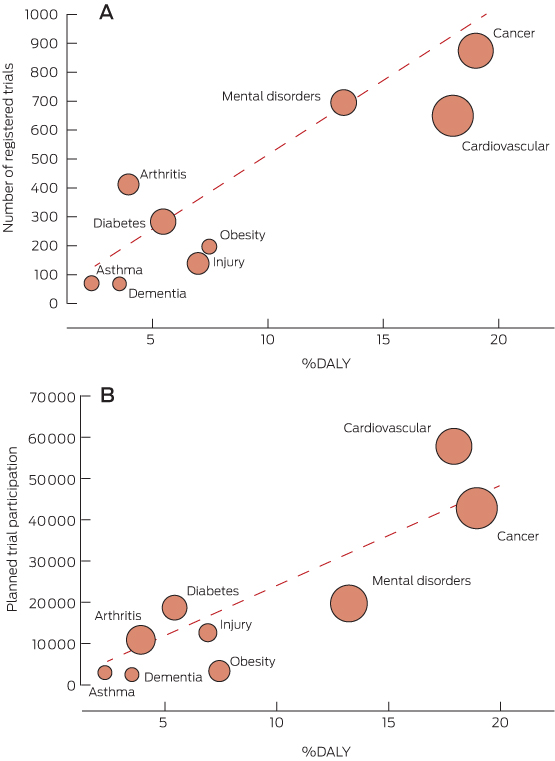
The diagonal line represents the line of equality where %DALY is equal to trial number as a percentage of total registered trials (A) or planned trial participation as % of total planned trial participation (B). Dots below the line show NHPAs where the variable falls below the %DALY. The size of dots corresponds to the size of planned trial participation (A) or number of trials (B) for the NHPA.
%DALY = disability-adjusted life-years expressed as a proportion of the total burden of disease in Australia.1 NHPA = National Health Priority Area.
3 Australian intervention trial characteristics, overall and by National Health Priority Area (NHPA),* Australian New Zealand Clinical Trials Registry and ClinicalTrials.gov, 2008–2012
Characteristic | All trials | NHPA | Cancer | Cardio- | Mental | Obesity | Injury | Diabetes | Arthritis/ | Dementia | Asthma | ||||
Total | 5143 | 3032 | 871 | 646 | 693 | 195 | 137 | 282 | 410 | 65 | 68 | ||||
Randomisation | |||||||||||||||
Yes | 3990 (78%) | 2335 (77%) | 564 (65%) | 494 (77%) | 579 (84%) | 163 (84%) | 125 (91%) | 253 (90%) | 321 (78%) | 53 (82%) | 59 (87%) | ||||
No | 1137 (22%) | 691 (23%) | 304 (35%) | 150 (23%) | 113 (16%) | 31 (16%) | 12 (9%) | 28 (10%) | 89 (22%) | 12 (18%) | 9 (13%) | ||||
Missing | 16 | 6 | 3 | 2 | 1 | 1 | 1 | ||||||||
Intervention type | |||||||||||||||
Treatment | 3834 (75%) | 2321 (76%) | 732 (84%) | 444 (69%) | 494 (71%) | 108 (55%) | 103 (75%) | 210 (75%) | 357 (87%) | 50 (77%) | 46 (68%) | ||||
Prevention | 781 (15%) | 397 (13%) | 52 (6%) | 131 (20%) | 98 (14%) | 67 (34%) | 25 (18%) | 46 (16%) | 34 (8%) | 5 (8%) | 10 (15%) | ||||
Diagnosis | 152 (3%) | 78 (3%) | 29 (3%) | 26 (4%) | 11 (2%) | 3 (2%) | 2 (2%) | 8 (3%) | 4 (1%) | 4 (6%) | 0 | ||||
Educational/ | 263 (5%) | 171 (6%) | 39 (5%) | 26 (4%) | 73 (11%) | 10 (5%) | 4 (3%) | 15 (5%) | 9 (2%) | 5 (8%) | 7 (10%) | ||||
Other/missing | 113 (2%) | 65 (2%) | 19 (2%) | 19 (3%) | 17 (2%) | 7 (4%) | 3 (2%) | 3 (1%) | 6 (2%) | 1 (2%) | 5 (7%) | ||||
Age group (years) | |||||||||||||||
Minimum age < 18† | 987 (19%) | 490 (16%) | 122 (14%) | 60 (9%) | 156 (23%) | 29 (15%) | 42 (31%) | 28 (10%) | 57 (14%) | 7(11%) | 26 (38%) | ||||
Missing | 5 | 2 | 1 | 1 | |||||||||||
Maximum age ≥ 70† | 3652 (71%) | 2252 (75%) | 774 (89%) | 558 (87%) | 397 (57%) | 69 (36%) | 98 (72%) | 199 (71%) | 316 (77%) | 59 (94%) | 41 (60%) | ||||
Missing | 18 | 10 | 2 | 2 | 1 | 2 | 2 | ||||||||
Blinding | |||||||||||||||
Blinded | 2639 (53%) | 1504 (51%) | 270 (31%) | 347 (55%) | 405 (61%) | 93 (51%) | 89 (67%) | 141 (52%) | 249 (64%) | 47 (72%) | 48 (72%) | ||||
Open | 2322 (47%) | 1427 (49%) | 589 (69%) | 281 (45%) | 260 (39%) | 91 (49%) | 43 (33%) | 129 (48%) | 139 (36%) | 18 (28%) | 19 (28%) | ||||
Missing | 182 | 101 | 12 | 18 | 28 | 11 | 5 | 12 | 22 | 0 | 1 | ||||
Planned recruitment | |||||||||||||||
1–100 | 2689 (52%) | 1509 (50%) | 361 (41%) | 325 (50%) | 361 (52%) | 132 (68%) | 66 (48%) | 133 (47%) | 228 (56%) | 22 (35%) | 33 (49%) | ||||
101–1000 | 2066 (40%) | 1274 (42%) | 427 (49%) | 244 (38%) | 300 (43%) | 58 (30%) | 61 (45%) | 119 (42%) | 161 (39%) | 35 (55%) | 31 (46%) | ||||
> 1000 | 383 (7%) | 246 (8%) | 83 (10%) | 77 (12%) | 30 (4%) | 5 (2%) | 10 (7%) | 30 (11%) | 21 (5%) | 6 (10%) | 3 (5%) | ||||
Missing | 5 | 3 | 1 | 2 | 2 | 1 | |||||||||
Country of recruitment | |||||||||||||||
Australia only | 3521 (68%) | 1951 (64%) | 349 (40%) | 401 (62%) | 578 (83%) | 184 (94%) | 113 (82%) | 192 (68%) | 286 (70%) | 37 (57%) | 47 (69%) | ||||
Australia and overseas | 1622 (32%) | 1081 (36%) | 522 (60%) | 245 (38%) | 115 (17%) | 11 (6%) | 24 (18%) | 90 (32%) | 124 (30%) | 28 (43%) | 21 (31%) | ||||
Data are no. (%) unless otherwise specified. * Trials may be classified under more than one NHPA (eg, obesity and diabetes). † Includes trials that did not specify age limits.
Received 22 April 2014, accepted 18 November 2014
- Jacquelyne Lam1
- Sarah J Lord1
- Kylie E Hunter1
- R John Simes1
- Thuyen Vu1
- Lisa M Askie1
- NHMRC Clinical Trials Centre, Sydney, NSW.
The ANZCTR is funded by an NHMRC enabling grant, Therapeutic Innovation Australia, and the Health Research Council of New Zealand.
No relevant disclosures.
- 1. Australian Institute of Health and Welfare, Begg S, Vos T, Barker B, et al. The burden of disease and injury in Australia 2003. Canberra: AIHW, 2007. (AIHW Cat. No. PHE 82.) http://www.aihw.gov.au/publication-detail/?id=6442467990 (accessed Apr 2012).
- 2. Aoun S, Pennebaker D, Pascal R. To what extent is health and medical research funding associated with the burden of disease in Australia? Aust N Z J Public Health 2004; 28: 80-86.
- 3. Mitchell RJ, McClure RJ, Olivier J, Watson WL. Rational allocation of Australia's research dollars: does the distribution of NHMRC funding by National Health Priority Area reflect actual disease burden? Med J Aust 2009; 191: 648-652. <MJA full text>
- 4. National Health and Medical Research Council, Australian Research Council, Australian Vice-Chancellors' Committee. National statement on ethical conduct in human research. Canberra: Australian Government, 2007. www.nhmrc.gov.au/_files_nhmrc/publications/attachments/e72.pdf (accessed Mar 2013).
- 5. Australian New Zealand Clinical Trial Registry. Data item definition/explanation. http://www.anzctr.org.au/docs/ANZCTR%20Data%20field%20explanation.pdf (accessed Apr 2012).
- 6. Tai F, Ooi W, Hunter K, et al. Data completeness and quality: the results of the Australian New Zealand Clinical Trial Registry 2011 data audits. Cochrane Database Syst Rev 2012; Suppl 1: 13. http://www.cochranelibrary.com/dotAsset/d5356a06-cddf-42c5-bf24-af7a6edbff99.pdf (accessed Jun 2015).
- 7. Califf RM, Zarin DA, Kramer JM, et al. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA 2012; 307: 1838-1847.
- 8. Dear RF, Barratt AL, McGeechan K, et al. Landscape of cancer clinical trials in Australia: using trial registries to guide future research. Med J Aust 2011; 194: 387-391. <MJA full text>
- 9. Clinical Trials Action Group. Clinically competitive: boosting the business of clinical trials in Australia. Clinical Trials Action Group Report. Canberra: Commonwealth of Australia, 2011. http://www.industry.gov.au/industry/IndustrySectors/PharmaceuticalsandHealthTechnologies/ClinicalTrialsActionGroup/Documents/Clinical_Trials_Action_Group_Report.pdf (accessed Apr 2014).
- 10. Winship IM, McNeil J, Simes RJ. A funding model for public-good clinical trials. Med J Aust 2013; 199: 90-91. <MJA full text>
- 11. Giannakakis IA, Haidich AB, Contopoulos-Ioannidis DG, et al. Citation of randomized evidence in support of guidelines of therapeutic and preventive interventions. J Clin Epidemiol 2002; 55: 545-555.





Rachelle Buchbinder
According to the WHO Global Burden of Disease (GBD) 2010 study, musculoskeletal conditions rank second after cancer (15.3% versus 16.2%), in their relative contribution to total disease burden in Australia (3). We therefore performed a reanalysis of the data presented by Lam et al., using the latest disease burden estimates from the WHO GBD study, which are publicly available as a series of data visualisations (4). Importantly, these estimates are continuously updated as further data become available.
The reanalysis changes the conclusions for three NHPAs. While cancer trials now match disease burden (16.9% of trials; 16.5% disease burden (% total DALYs) according to GBD2010 vs 19.0% AIHW 2003) and diabetes trials more than twice exceed expected numbers (5.5% of trials; 2.3% disease burden GBD2010 vs 5.5% AIHW 2003), the proportion of registered arthritis and musculoskeletal trials is now about half that expected based upon their relative disease burden (8% of trials; 14.7% disease burden GBD2010 vs 4.0% AIHW 2003), indicating a significant (and growing) area of need. The recently established Australia & New Zealand Musculoskeletal (ANZMUSC) Clinical Trials Network aims to address this gap by facilitating high quality collaborative clinical trials focused upon optimising musculoskeletal health.
References
1. Lam J, Lord SJ, Hunter KE, et al. Australian clinical trial activity and burden of disease: an analysis of registered trials in National Health Priority Areas. Med J Aust 2015; 203: 97-102.
2. Bourne AM, Whittle SL, Richards BL, Maher CG, Buchbinder R. The scope, funding and publication of musculoskeletal clinical trials performed in Australia. Med J Aust 2014; 200: 88-91.
3. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197-2223.
4. GBD 2010 Country Collaboration. GBD 2010 country results: a global public good. Lancet 2013; 381: 965–968.
Competing Interests: No relevant disclosures
Prof Rachelle Buchbinder
Monash University and Cabrini Institute
Sarah JLord
The updated relative burden of disease estimates for musculoskeletal conditions highlight the importance of reviewing research priorities over time given changes in disease incidence, prevalence and mortality, and ongoing improvements in methods for the estimation of disease burden. In addition to the GBD data available from the Institute for Health Metrics and Evaluation, in 2014 the World Health Organisation (WHO) published 2012 burden of disease estimates for Australia (available at: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html). Despite differences in the relative burden of disease estimates for musculoskeletal conditions from these two sources, both are higher than the AIHW 2007 estimates used in our study.
We hope the public availability of trial registry data will similarly encourage other researchers to explore research gaps in other disease areas to better inform research programs, policy makers, and funders about research priorities. Following our study period end date in December 2012 (1), over 3600 new trials with Australian sites have been added to the WHO International Clinical Trials Registry Platform (available at: http://apps.who.int/trialsearch) which receives weekly uploads from the Australian New Zealand Clinical Trials Registry and 15 other trial registries, allowing public access to up-to-date trial data.
References
1. Lam J, Lord SJ, Hunter KE, et al. Australian clinical trial activity and burden of disease: an analysis of registered trials in National Health Priority Areas. Med J Aust 2015; 203: 97-102.
Competing Interests: No relevant disclosures
Dr Sarah JLord
NHMRC Clinical Trials Centre, The University of Sydney