Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide.1 Guidelines for care of COPD provide recommendations for slowing disease progression and optimising function in people with COPD.2,3 The key interventions are smoking cessation, pulmonary rehabilitation, influenza vaccination, optimising medicines, patient education and effective management of exacerbations.
A review of nine randomised trials of nurse-led chronic disease management for COPD concluded that there was no evidence of improvements in patients’ health-related quality of life, psychological wellbeing, disability or pulmonary function.4 A more recent Cochrane review of nine trials of outreach programs involving nurse home visits to COPD patients concluded that providing support and education, monitoring health status and providing liaison with physicians resulted in improved disease-specific quality-of-life measures but had variable effects on hospitalisation.5 A New Zealand study, which was not included in the Cochrane Review as it did not have a substantial home-visit component, resulted in reduced hospital admissions and bed-days, and significant improvements in quality of life and lung function.6
A previous randomised trial conducted by members of our group evaluated the effect of a brief nurse-led intervention, including development of a care plan, after discharge from hospital on clinical outcomes in patients with COPD.7 There was no difference between groups in health-related quality of life or hospital admissions. Patients in the intervention group had higher knowledge scores and were more satisfied with their care. In that study, less than a third of the general practitioners remembered receiving the care plan, and there were no differences in GP visits or management.
A cluster randomised trial, with randomisation at the level of the practice, was conducted to avoid contamination between intervention and control groups. The study protocol has been published.8 Recruitment started in December 2006 and follow-up was completed in May 2009. Ethics approval was from University of New South Wales and Sydney South West Area Health Service human research ethics committees.
A researcher who took no further part in the study randomised practices to intervention or control groups, with allocation concealment. Details of the randomisation process have been published previously.8
Two nurses, specifically recruited and trained for this study, worked in partnership with GPs to implement the intervention. In the service model, the nurses were external to the practice and visited patients in their homes.6 The training program for the nurses involved attendance at a 2-day workshop where the following topics were presented by expert clinicians: patho-physiology of COPD; assessment of COPD; spirometry; smoking cessation; management of COPD according to Australian and New Zealand guidelines;3 the role of pulmonary rehabilitation in the management of COPD; and the management of exacerbations. The training covered the principles and practice of motivational interviewing and self-management support. Following the training, there were monthly meetings lasting 1–2 hours between the nurses and members of the study team (N Z and S V), and feedback from a respiratory physician on the quality of their spirometry (G M). The intervention and its implementation are described in theAppendix. The intervention was delivered between 2007 and 2009.
The primary outcome measure was disease-related quality of life, measured using the St George’s Respiratory Questionnaire (SGRQ) at 12 months after recruitment. The SGRQ is a validated instrument designed to measure the impact of respiratory diseases (in particular, asthma and COPD) on an individual’s life.9 The SGRQ is scored from zero to 100, where zero indicates best quality of life and 100, the worst. A change in score of ≥ 4 is considered to be clinically significant.9,10
Other outcome measures were overall quality of life (measured using the 12-item Short Form Health Survey [SF-12], which is a generic measure of health impairment); lung function; smoking status; immunisation status for influenza and pneumococcus; attendance at pulmonary rehabilitation; patient knowledge of COPD; and health service use. For those patients with COPD on spirometry, classification of severity was made using Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.2 The patient’s knowledge of COPD was measured by a 12-item scale developed for a previous project.7 This scale asked patients if they knew the name of their chest condition, which immunisations were helpful in reducing the risk of exacerbations, patient actions that could help control symptoms and improve quality of life, and symptoms suggestive of an exacerbation. Correct answers scored one point and incorrect answers zero points, resulting in a score out of 12. The scale has face validity but has not been subjected to validation testing.
The sample size calculation was based on detecting a between-group difference in SGRQ score of ≥ 4 at 12 months after intervention.9,10 After adjusting for clustering, the number per group required to detect this difference with 80% power at the 5% significance level was estimated to be 200 per group, based on an intracluster coefficient of 0.01 and a resultant design effect of 1.09 for a cluster size of 10. Details of the sample size calculation have been published.8
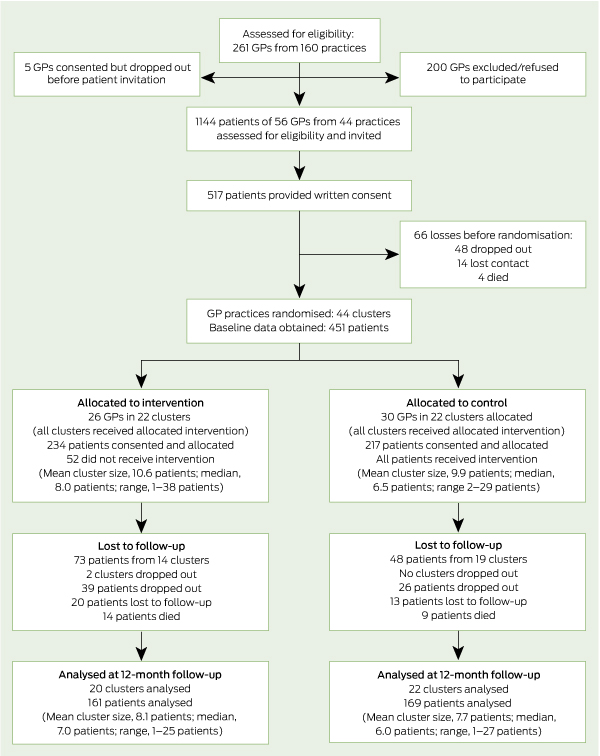
We recruited 56 GPs from 44 practices in south-west Sydney. The mean age of the GPs was 52.3 years and 47% were men. Participating GPs searched their patient records, identifying 1144 patients who were eligible and invited to participate. Of these, 451 (39.4%) patients were recruited and provided baseline data, 330 (73.2%) of whom completed the 12-month assessment (see flow diagram). Characteristics of patients completing the 12-month assessment compared with those lost to follow-up were: age, 65.3 v 64.7 years; men, 47.3% v 49.6%; spoke English at home, 79.1% v 79.7%; and current smokers, 30.2% v 31.1%. There was a lower rate of confirmed COPD than expected. Of the 451 participants, 445 (98.7%) were able to perform baseline spirometry and, of these, 257 (57.8%) were confirmed to have COPD.11
Baseline characteristics of participants are shown in Box 1. There was a higher rate of confirmed COPD, lower forced expiratory volume in 1 second and fewer comorbidities in the intervention group, but the groups did not differ on the SGRQ or other characteristics.
There were no statistically significant between-group differences in the primary outcome measure (SGRQ) or in overall quality of life, respiratory function or smoking status at 12-month follow-up (Box 2). The intracluster correlation for the SGRQ calculated using the mixed-model procedure was 0.03. In continuing smokers, there was a trend towards fewer cigarettes smoked per day in the intervention group.
In process-of-care outcomes, more patients in the intervention group reported having attended a pulmonary rehabilitation program. This difference was statistically significant (P = 0.002). Data were not collected on whether they completed the program. There was a higher rate of patients in the intervention group reporting being vaccinated for influenza and pneumococcus, but neither difference was statistically significant. There was no difference in the frequency of either GP or hospital or emergency department attendance in the previous 3 months. The COPD knowledge score was statistically significantly higher in the intervention group (P = 0.02) (Box 3).
A notable finding was that only 57.8% of patients identified as having COPD and being eligible for the study were confirmed as having the condition according to spirometric criteria. We have previously published baseline analyses of the accuracy of diagnosis showing that having a spirometer in the practice was not predictive of agreement between the clinical and spirometric diagnoses.11 Older patient age was associated with correct diagnosis, while higher numbers of comorbidities were associated with misdiagnosis.
There was greater attendance at a pulmonary rehabilitation program by patients in the intervention group, indicating improved delivery of evidence-based care. It has been shown that participation in pulmonary rehabilitation leads to improvements in health-related quality of life (measured by the SGRQ) at the end of the program.12 The fact that these benefits were not apparent in our study may be due to insufficient numbers of participants attending and/or completing pulmonary rehabilitation, or due to improvements evident at the end of the program not being sustained to the outcome measurements points. The higher rate of influenza and pneumococcal vaccination in the intervention compared with the control group suggests improved delivery of care, but these differences were not statistically significant. Patients in the intervention group had higher COPD knowledge scores, presumably related to the education provided by the nurses, although the clinical importance of this is uncertain.
The criterion for entry into the study was having a diagnosis of COPD and we did not require this to be confirmed on spirometry. This reflects the real-world situation in primary care, where the diagnosis is often made and treatment initiated on clinical grounds.13-15 The rate of misclassification was similar to that of other studies from Australia16 and internationally.17,18
However, the intervention components were based on evidence of effectiveness from studies in patients with COPD confirmed on spirometry, and the intervention may only have been effective in this subgroup of participants. A subgroup analysis, which examined the outcomes for the 257 patients who had COPD confirmed on spirometry, was similar to the intention-to-treat analysis, with no statistically significant differences at 12 months in SGRQ, SF-12, lung function or smoking rates. As the numbers were smaller, there was a risk of a type 2 error, and this risk was further increased as the intracluster correlation found for the SGRQ (0.03) was slightly higher than our estimate of 0.01.18
Our findings are consistent with the current uncertainty about the effect of disease-management programs, including self-management support, for COPD. While some studies have shown benefit,6,19 others, including a recently published study of comprehensive care management to prevent COPD hospitalisations, have had negative results.20
The lack of impact from the intervention on prevalence of smoking demonstrates the need to continue to develop and test interventions to encourage smoking cessation in people with COPD. There is continuing debate about whether performing spirometry and informing patients of abnormal results increases smoking cessation.21,22 There has been promising research on the use of lung age as a tool to encourage quitting, but this has not been studied in patients with COPD.23 The evidence base on smoking-cessation interventions for people with COPD is very limited,24 and there is a need for studies that evaluate both psychosocial approaches and innovative ways of using pharmacotherapy.25
Pulmonary rehabilitation has the potential to improve health-related quality of life, but even in the intervention group, less than a third of patients attended pulmonary rehabilitation. This was consistent with previous research reporting uptake of 33%–39% in pulmonary rehabilitation programs provided in outpatient clinics.26 There is evidence that home-based programs may be as effective as supervised hospital outpatient-based programs,27,28 but studies on implementation are lacking. Finally, there is the question of whether the nurse–GP partnership intervention to implement evidence-based care would have been effective if it had been implemented with patients at an early stage of the disease. It has been suggested, for example, that the benefit of smoking cessation may be greatest in asymptomatic patients with measureable lung function impairment.29
2 Disease-related and overall quality of life, respiratory function and smoking status at 12-month follow-up
3 Process-of-care outcomes at 12-month follow-up
No. used hospital or emergency department service in preceding 3 months (%) |
|||||||||||||||
(unedited, as supplied by the authors)
- An initial home visit involving comprehensive assessment, including pre and post bronchodilator spirometry.
- Development of a personalised care plan based on the recommendations of the COPDX guidelines using an electronic template provided. The care plan was based on the nurse assessment and discussion with the patient of goal setting and action planning. It the contained relevant components of smoking cessation, influenza and pneumococcal immunisation, pulmonary rehabilitation, medication review, nutrition, psychosocial issues, patient education, comorbidities and complications of COPD. Where spirometry did not confirm COPD the nurse discussed with the GP the actions following this and what parts of the care plan applied to these patients.
- The nurse worked with the patient, their GP and other health professionals to implement the plan. This involved at least two home visits and five telephone contacts from the nurse and a minimum of two consultations with their GP. The nurse facilitated referral and teamwork with other services as needed such as smoking cessation program, pulmonary rehabilitation program, pharmacist, specialist physician, Action Plans for exacerbations were discussed and patients were encouraged to take these to their GPs for completion.
- At the end of the six month intervention period progress against the goals in the plan were noted and a copy of the plan with these annotations provided to the GP. The completion of the plan was used to define that the patient had received the intervention.
Received 17 May 2012, accepted 23 August 2012
- Nicholas A Zwar1
- Oshana Hermiz1
- Elizabeth Comino1
- Sandy Middleton2
- Sanjyot Vagholkar1
- Wei Xuan3
- Stephen F Wilson4
- Guy B Marks3
- 1 University of New South Wales, Sydney, NSW.
- 2 Centre for Clinical Outcomes Research, Australian Catholic University, Sydney, NSW.
- 3 Woolcock Institute of Medical Research, Sydney, NSW.
- 4 Department of Rehabilitation Medicine, Royal North Shore Hospital, Sydney, NSW.
We acknowledge the contribution of Iqbal Hasan to data collection, the nurses, GPs and patients who participated in the study, and the Divisions of General Practice who supported the study. Funding was from the National Health and Medical Research Council.
No relevant disclosures.
- 1. Price D, Duerden M. Chronic obstructive pulmonary disease. BMJ 2003; 326: 1046-1047.
- 2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. Updated Dec 2011. http://www.goldcopd.org (accessed Aug 2012).
- 3. McKenzie DK, Abramson M, Crockett AJ, et al; The Australian Lung Foundation. The COPD-X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2011. http:www.copdx.org.au (accessed Aug 2012).
- 4. Taylor SJ, Candy B, Bryar RM, et al. Effectiveness of innovations in nurse led chronic disease management for patients with chronic obstructive pulmonary disease: systematic review of evidence. BMJ 2005; 331: 485.
- 5. Wong CX, Carson KV, Smith BJ. Home care by outreach nursing for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011; (3): CD000994.
- 6. Rea H, McAuley S, Stewart A, et al. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004; 34: 608-614.
- 7. Hermiz O, Comino E, Marks G, et al. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002; 325: 938.
- 8. Zwar N, Hermiz O, Hasan I, et al. A cluster randomised controlled trial of nurse and GP partnership for care of chronic obstructive pulmonary disease. BMC Pulm Med 2008; 8: 8.
- 9. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321-1327.
- 10. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19: 217-224.
- 11. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust 2011; 195: 168-171. <MJA full text>
- 12. Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; (4): CD003793.
- 13. Walters JA, Hansen E, Mudge P, et al. Barriers to use of spirometry in general practice. Aust Fam Physician 2005; 34: 201-203.
- 14. Poels PJP, Schermer TRJ, van Weel C, Calverley PMA. Spirometry in chronic obstructive pulmonary disease. BMJ 2006; 333: 870-871.
- 15. Walters J, Walters EH, Nelson M, et al. Factors associated with misdiagnosis of COPD in primary care. Prim Care Respir J 2011; 20: 396-402
- 16. Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma 2006; 43: 75-80.
- 17. Jones RC, Dickson-Spillmann M, Mather MJ, et al. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respir Res 2008; 9: 62.
- 18. Kerry SM, Bland JM. Trials which randomize practices II: sample size. Family Practice 1998; 15: 84-87.
- 19. Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003; 163: 585-591.
- 20. Fan VS, Gaziano M, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations. Ann Int Med 2012; 156: 673-683.
- 21. Wilt TJ, Niewoehner D, Kane RL, et al. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res 2007; 9: 21-32.
- 22. Kotz D, Wesseling G, Huibers MJ, van Schayck OC. Efficacy of confronting smokers with airflow limitation for smoking cessation. Eur Respir J 2009; 33: 754-62.
- 23. Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008; 336: 598-600.
- 24. van der Meer RM, Wagena EJ, Ostelo RW, et al. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003; (2): CD002999.
- 25. Moore D, Aveyard P, Connock M, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009; 338: b1024.
- 26. Harris D, Hayter M, Allender S. Improving the uptake of pulmonary rehabilitation in patients with COPD: qualitative study of experiences and attitudes. Br J Gen Pract 2008; 58: 703-710.
- 27. Ashworth NL, Chad KE, Harrison EL, et al. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev 2005; (1): CD004017.
- 28. Maltais F, Bourbeau J, Shapiro S, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2008; 149: 869-878.
- 29. Price D, Freeman D, Cleland J, et al. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J 2011; 20: 15-22.





Abstract
Objective: To evaluate a partnership model of care for patients with a diagnosis of chronic obstructive pulmonary disease (COPD).
Design, setting and participants: Cluster randomised controlled trial with blinded outcome assessment of 44 general practices in south-western Sydney comprising 451 people with a diagnosis of COPD, conducted between 2006 and 2009.
Intervention: Participants from intervention group practices were visited at their home by a registered nurse with specific training in COPD care who worked with the general practitioner, the patient and other health professionals to develop and implement an individualised care plan based on best-practice guidelines. Participants from control group practices received usual care.
Main outcome measures: The primary outcome was disease-related quality of life measured using the St George’s Respiratory Questionnaire (SGRQ) at 12-month follow-up. Other outcomes were overall quality of life, lung function, smoking status, immunisation status, patient knowledge of COPD, and health service use.
Results: Of the 451 participants, 257 (57.8%) were confirmed as having COPD on post-bronchodilator spirometry. Follow-up was completed for 330 patients (73.2%). At 12 months, there was no statistically significant difference in the mean SGRQ scores between intervention and control groups (38.7 v 37.6; difference, 1.1; 95% CI, − 1.53–3.74; P = 0.41) or in measures of quality of life, lung function and smoking status. Compared with the control group, in the intervention group, attendance at pulmonary rehabilitation was more frequent (31.1% v 9.6%; OR, 5.16; 95% CI, 2.40–11.10; P = 0.002) and the mean COPD knowledge score was higher (10.5 v 9.8; difference, 0.70; CI, 0.10–1.21; P = 0.02).
Conclusion: The nurse–GP partnership intervention did not have an impact on disease-related quality of life at 12-month follow-up. However, there was evidence of improved quality of care, in particular, in attendance at pulmonary rehabilitation and patient knowledge of COPD.
Trial registration: Australian Clinical Trials Registry ACTRN012606000304538.