Infection surveillance is a crucial component of an integrated infection control program, providing timely feedback about infection prevention issues that is geared towards decreasing the burden of infection within health care facilities.1 Data from overseas suggest there is a considerable infection burden among aged care residents.2-6 The Aged Care Standards and Accreditation Agency Ltd has highlighted that surveillance is required in Australian residential aged care facilities (RACFs), but there are few published studies describing the epidemiology of health care-associated infection (HCAI) and antimicrobial use.7,8
A recent Australian study highlighted a concern about emergence of antimicrobial-resistant organisms among RACF residents.8 The study, however, did not describe the infection burden and the associated antimicrobial prescribing patterns among this population. Although some overseas studies report extensive prescribing of broad-spectrum antibiotics,9-11 differences in clinical practice are likely in Australia.
Accordingly, we explored the patterns of common infections and use of antimicrobial agents in four Australian RACFs using internationally recognised criteria.12 Recognition of the infection burden among residents in this long-term care setting will help to identify infection prevention priorities and guide antimicrobial stewardship efforts.
A systematic infection surveillance system was introduced across the RACFs; initially (from 2003), to monitor the incidence of urinary tract infection (UTI) and conjunctivitis, then later (from mid 2005), other HCAIs were included. HCAI refers to infection occurring at least 48 hours after admission to an RACF with new or acutely worsening symptoms that fulfil criteria for site-specific infection. These definitions, known as the McGeer criteria,12 refer to a set of clinical and other criteria intended specifically for use in long-term care facilities (See appendix). They have been widely accepted as an epidemiological tool for interfacility comparison of infection rates, both locally and globally.3,5,7,13,14
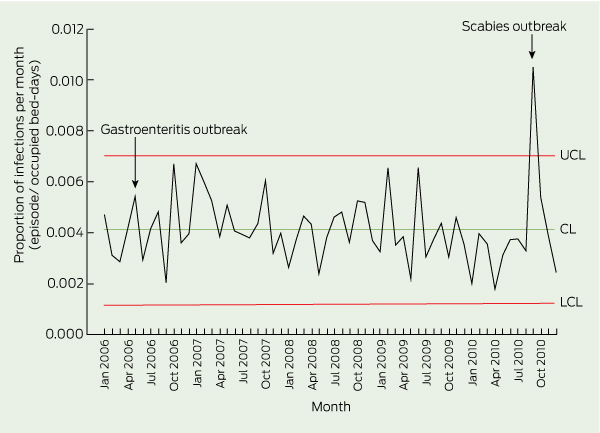
We used descriptive statistics to summarise the data — number and percentage for categorical variables, and mean for continuous variables. Rate of infection was calculated as the number of episodes of infections per 1000 occupied bed-days (OBD), whereas antimicrobial prescribing was described in courses per 1000 OBD. We calculated the 95% confidence interval (CI) for the infection rate using OpenEpi version 2.3.1 (Open Source Epidemiologic Statistics for Public Health, version 2.3.1; http://www. openepi.com), assuming a Poisson distribution. We used the monthly recorded rates of infection that conformed to the McGeer criteria over the 5-year period to construct a statistical control chart, using IBM SPSS Statistics 19 (IBM Corporation, New York, NY, USA). A proportion (p) control chart was selected to account for the proportion of infections in the studied RACF population over time.15 Infection rates above the upper control limit may indicate a potential outbreak.
Over 5 years, from January 2006 to December 2010, 1114 infections that fulfilled the McGeer criteria were recorded over 267 684 OBD (98% bed occupancy) in the four RACFs studied. The estimated mean HCAI rate was 4.16 episodes per 1000 OBD annually (95% CI, 3.92–4.41). The infection rates across the four RACFs were similar, with yearly incidence ranging from 3 to 5.5 episodes per 1000 OBD. The distribution of validated HCAIs is shown in Box 1.
The monthly trend of infections within the population was assessed using a p control chart (Box 2); the monthly proportion significantly exceeded the upper control limit in September 2010, owing to a scabies outbreak. While the statistical control chart suggested some fall in infection rates at the end of the study (eg, with eight points below the mean, January 2010 to August 2010), the overall trend in the rate of infections showed a small non-significant decrease over the 5-year period.
Between 2009 and 2010, the mean rate of antimicrobial use was 7.07 courses per 1000 OBD (range, 6.71–7.84 courses per 1000 OBD for the four RACFs). A total of 755 courses of antimicrobials were prescribed, of which 86.2% were administered orally, 9.4% were administered topically and 4.4% were given parenterally. Almost all were antibacterials; 1.5% were antiviral or antifungal agents.
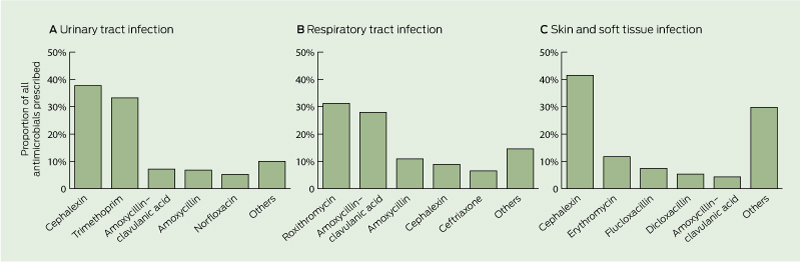
Cephalexin, trimethoprim, amoxycillin–clavulanic acid and roxithromycin accounted for 62% of all the courses prescribed (Box 3). UTI was the most common indicator for antimicrobial use (in 41% of courses), followed by LRTI (in 26%), SSTI (in 12%), URTI (in 8%) and eye infection (in 8%). Box 4 shows antimicrobial prescribing patterns for three major sites of infection (ie, UTI, LRTI and URTI combined, and SSTI). Only about 5% of antimicrobials were prescribed for other indications such as ear and mouth infection, gastroenteritis, bloodstream infection and unexplained febrile illness. Around 11% of all antimicrobials prescribed for UTIs were broad-spectrum antibiotics, which included norfloxacin, ciprofloxacin, ceftriaxone and gentamicin. Indwelling catheter-related (IDC) UTI contributed to about 20% of all episodes of UTI; prescribing patterns for both non-IDC and IDC UTIs were similar. Antimicrobials were routinely prescribed for URTI and bronchitis (constituting up to 31% of overall antimicrobial use). Similar prescribing patterns were observed for episodes that fulfilled McGeer criteria and those that did not.
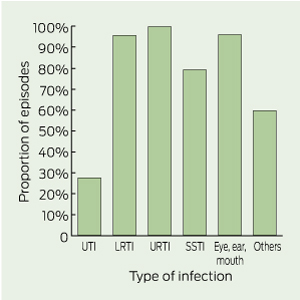
Of 659 episodes for which patients were treated with antimicrobials, only 238 episodes (36%) had documentation that clinical specimens were obtained. Clinical specimens were infrequently collected for most types of infection (Box 5). Conversely, collection of urine specimens was documented for 72.2% of UTI episodes.
Our results indicate that systematic infection surveillance can be established and sustained in Australian RACFs using internationally established criteria,12 although there are currently no systematic surveillance systems for infection in RACFs in any Australian jurisdiction. The only two published Australian studies that have examined HCAI rates in this setting were conducted over a 9-year period in a Sydney network and as a 6-month pilot study in a rural Victorian region.7,16 Both studies used the McGeer criteria and reported rates of infection at 3.1 and 2.2 infections per 1000 OBD, respectively. These rates are of the same magnitude as infection rates reported in studies from the United States and European countries of 1.5–9.5 infections per 1000 OBD, based on the McGeer criteria.2-6,14 The average rate and types of HCAI in our study were generally comparable to the reported literature, except for the rate of pneumonia, which was considerably lower than that in some studies.11,17,18 We found that chest x-rays, required to fulfill criteria for pneumonia, were often not performed in this patient population, suggesting that the low rate may reflect differences in clinical practice, rather than a true difference in pneumonia rate. The rate of gastroenteritis (0.15 episodes per 1000 OBD) was lower than that reported in one other local study (0.29–1.42 episodes per 1000 OBD).19
There was a significant burden of oral antimicrobial use in this population, with a high proportion of treated episodes not meeting infection criteria, reflecting the difficulties in clinically assessing this patient popula-tion. The antimicrobial burden in the RACFs we studied (7.07 courses per 1000 OBD) was mid-range compared with those reported in overseas studies (2–15 courses per 1000 OBD).10,17,20,21 Data from Australian RACFs for benchmarking are lacking. The rate of antimicrobial use cannot easily be compared with hospital-based and community-based studies, owing to differences in surveillance methods. Nonetheless, if the average duration of an antimicrobial course was around 7 days, the use rate could be converted and would approximate 50 defined daily doses (DDD) per 1000 OBD, which is higher than the estimated use in the community (about 25 DDD/1000 population days)22 but much lower than the estimated use in large Australian hospitals (about 1000 DDD/1000 OBD).23
In our study, the considerable proportion of antimicrobials prescribed for episodes that did not meet the McGeer criteria was similar to that reported elsewhere.8,20 Although the McGeer criteria are well established for epidemiological comparison, antimicrobial use in patients not meeting these criteria may not always be inappropriate. Nevertheless, it was noted that all episodes of URTI and nearly all of bronchitis led to routine prescribing of antimicrobials without further diagnostic investigation to confirm bacterial aetiology, contrary to recommendations in the national Antibiotic Therapeutic Guidelines.24 Similarly, the use of antimicrobials for asymptomatic bacteriuria is not recommended. The prescribing of antimicrobials for asymptomatic bacteriuria is of particular concern because the emergence of multiresistant organisms in the RACF setting25,26 is often attributable to extensive or inappropriate use of antimicrobials and may result in the RACFs becoming a reservoir for multiresistant organisms. Barriers to improving the quality of antimicrobial prescribing include the complexities of assessing elderly patients due to atypical illness presentation, lack of timely investigations to guide therapy, and the large number of health care providers involved in the care of RACF residents.
Several sources suggest that fluoroquinolones were the most commonly prescribed antibiotics for common infections in the RACF setting overseas.9,10,27,28 However, our data show that cephalexin and trimethoprim were used to a much higher extent, and the use of fluoroquinolones or other broad-spectrum antibiotics was low. The low use of fluoroquinolones is likely to reflect, at least in part, restricted indications under the Australian Pharmaceutical Benefits Scheme. The high use of chloramphenicol, on the other hand, was due to the widespread prescribing of topical chloramphenicol eye preparations for conjunctivitis. Pleasingly, in most cases, the empirical prescribing pattern for UTIs was in line with the national guidelines.24
There were several limitations to our study. The results from four RACFs located on a hospital campus may not be generalisable to other RACF populations. However, we noted that most subjects were residents of the facilities, with little traffic of patients from the hospital. The rates of infection remained relatively constant and were comparable to those reported in another longitudinal study in Australia.7 Although the McGeer criteria are the only internationally recognised epidemiological definitions of infection, they may not be completely specific or sensitive for microbiologically confirmed infection. We were not able to analyse the epidemiology of bacterial pathogens, owing to the small number of cultures performed. Recent point prevalence studies have assisted in defining the prevalence of colonisation with antimicrobial-resistant pathogens,8 but data are also required on their contribution to the burden of infection to inform antibiotic prescribing policies.
1 Proportion and incidence rate of major types of health care-associated infection in four Melbourne RACFs, January 2006 to December 2010
Upper and lower respiratory tract infection (URTI† and LRTI‡) |
|||||||||||||||
2 Proportion control chart showing proportion of infections (episodes/OBD) per month in four Melbourne RACFs, January 2006 to December 2010 (n = 1114)
5 Proportion of episodes with no documentation of collection of a clinical specimen, by type of infection (n = 659)
Appendix The “McGeer criteria”* — definitions of infection for surveillance in long-term care facilities
Received 12 January 2012, accepted 22 February 2012
- Ching Jou Lim1
- Susan C McLellan2
- Allen C Cheng2,3
- Joanne M Culton2
- Sneha N Parikh4
- Anton Y Peleg*4,5
- David C M Kong*1
- 1 Centre for Medicine Use and Safety, Monash University, Melbourne, VIC.
- 2 Infection Prevention and Healthcare Epidemiology Unit, Alfred Health, Melbourne, VIC.
- 3 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 4 Infectious Diseases Unit, Alfred Hospital, Melbourne, VIC.
- 5 Microbiology Department, Monash University, Melbourne, VIC.
We thank Denis Spelman, Jacqueline Kennon, Kerrie Watson, the Alfred Infection Prevention and Hospital Epidemiology Unit, and Victorian Infection Control Surveillance Project. We also thank Andrew Perta, Gabrielle Melican and the nursing home managers (Kylie-Ann Thomas, Olga Rozjimalina, Galina Pudel and Wima Edwards) for their input.
No relevant disclosures.
- 1. Hughes JM. Study on efficacy of nosocomial infection control (SENIC Project): results and implications for the future. Chemotherapy 1988; 34: 553-561.
- 2. Darnowski SB, Gordon M, Simor AE. Two years of infection surveillance in a geriatric long-term care facility. Am J Infect Control 1991; 19: 185-190.
- 3. Engelhart ST, Hanses-Derendorf L, Exner M, Kramer MH. Prospective surveillance for healthcare-associated infections in German nursing home residents. J Hosp Infect 2005; 60: 46-50.
- 4. Eriksen HM, Koch AM, Elstrøm P, et al. Healthcare-associated infection among residents of long-term care facilities: a cohort and nested case-control study. J Hosp Infect 2007; 65: 334-340.
- 5. Roberts C, Roberts J, Roberts R. Survey of healthcare-associated infection rates in a nursing home resident population. J Infect Prev 2010; 11: 82-86.
- 6. Schulz M, Mielke M, Wischnewski N. Clusters of infectious diseases in German nursing homes: observations from a prospective infection surveillance study, October 2008 to August 2009. Euro Surveill 2011; 16. pii: 19881.
- 7. Forrest J, Tucker A, Brnabic AJM. A 9-year infection-control surveillance program in Sydney-based residential aged-care facilities. Healthcare Infection 2011; 16: 108-114.
- 8. Stuart RL, Kotsanas D, Webb B, et al. Prevalence of antimicrobial-resistant organisms in residential aged care facilities. Med J Aust 2011; 195: 530-533. <MJA full text>
- 9. Loeb MB, Craven S, McGeer AJ, et al. Risk factors for resistance to antimicrobial agents among nursing home residents. Am J Epidemiol 2003; 157: 40-47.
- 10. Mylotte JM, Keagle J. Benchmarks for antibiotic use and cost in long-term care. J Am Geriatr Soc 2005; 53: 1117-1122.
- 11. Pettersson E, Vernby A, Molstad S, Lundborg CS. Infections and antibiotic prescribing in Swedish nursing homes: a cross-sectional study. Scand J Infect Dis 2008; 40: 393-398.
- 12. McGeer A, Campbell B, Emor TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control 1991; 19: 1-7.
- 13. Moro ML, Mongardi M, Marchi M, Taroni F. Prevalence of long-term care acquired infections in nursing and residential homes in the Emilia-Romagna region. Infection 2007; 35: 250-255.
- 14. Stevenson KB, Moore J, Colwell H, Sleeper B. Standardized infection surveillance in long-term care: interfacility comparisons from a regional cohort of facilities. Infect Control Hosp Epidemiol 2005; 26: 231-238.
- 15. Benneyan JC. Statistical quality control methods in infection control and hospital epidemiology, part I: Introduction and basic theory. Infect Control Hosp Epidemiol 1998; 19: 194-214.
- 16. Smith M, Bull AL, Richards M, et al. Infection rates in residential aged care facilities, Grampians region, Victoria, Australia. Healthcare Infection 2011; 16: 116–120.
- 17. Loeb M, McGeer A, McArthur M, et al. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med 1999; 159: 2058-2064.
- 18. Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med 1998; 105: 319-330.
- 19. Kirk MD, Moffatt CRM, Hall GV, et al. The burden of infectious gastroenteritis in elderly residents and staff of long-term care facilities, Australia. Infect Control Hosp Epidemiol 2010; 31: 860-863.
- 20. Loeb M, Simor AE, Landry L, et al. Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med 2001; 16: 376–383.
- 21. Mylotte JM. Antimicrobial prescribing in long-term care facilities: prospective evaluation of potential antimicrobial use and cost indicators. Am J Infect Control 1999; 27: 10-19.
- 22. McManus P, Hammond ML, Whicker SD, et al. Antibiotic use in the Australian community, 1990-1995. Med J Aust 1997; 167: 124-127. <MJA full text>
- 23. National antimicrobial utilisation surveillance program: annual report 2009–2010. Department of Health, Government of South Australia. http://www.health.sa.gov.au/INFECTIONCONTROL/Default.aspx?PageContentID=65&tabid=199 (accessed Jan 2012).
- 24. Antibiotic Guidelines Sub-Committee. Therapeutic guidelines: antibiotic (version 14). Therapeutic Guidelines Limited and Victorian Drug Usage Advisory Committee, 2010. http://www.tg.org.au (accessed Jan 2012).
- 25. Lautenbach E, Marsicano R, Tolomeo P, et al. Epidemiology of antimicrobial resistance among gram-negative organisms recovered from patients in a multistate network of long-term care facilities. Infect Control Hosp Epidemiol 2009; 30: 790-793.
- 26. O’Fallon E, Pop-Vicas A, D’Agata E. The emerging threat of multidrug-resistant gram-negative organisms in long-term care facilities. J Gerontol A Biol Sci Med Sci 2009; 64: 138-141.
- 27. D’Agata E, Mitchell SL. Patterns of antimicrobial use among nursing home residents with advanced dementia. Arch Intern Med 2008; 168: 357-362.
- 28. Pakyz AL, Dwyer LL. Prevalence of antimicrobial use among United States nursing home residents: results from a national survey. Infect Control Hosp Epidemiol 2010; 31: 661-662.





Abstract
Objectives: To explore the burden of illness associated with infectious syndromes and to measure the associated use of antimicrobials in residential aged care facilities (RACFs).
Design, setting and subjects: Retrospective analysis of data for January 2006 to December 2010 from an infection surveillance system covering residents of four co-located RACFs, with a total of 150 residential care beds, in Melbourne, Victoria.
Main outcome measures: Number of episodes and incidence of health care-associated infection (HCAI); rate of antimicrobial use; prescribing concordance with McGeer criteria for infection; frequency of clinical specimen collection.
Results: There were 1114 episodes of an infectious syndrome over 267 684 occupied bed-days (OBD), affording an average HCAI rate of 4.16 episodes/1000 OBD annually over 5 years (95% CI, 3.92–4.41). The mean rate of antimicrobial use was 7.07 courses/1000 OBD (range, 6.71–7.84). Around 40% of antimicrobial prescribing was for episodes that did not fulfil the McGeer criteria for clinical infection; this included about half of suspected urinary tract and upper respiratory tract infections (URTI), and about one-third of suspected lower respiratory tract and skin infections. Antimicrobials were routinely prescribed for URTI and bronchitis. Of all episodes treated with antimicrobials, 36% had documentation that a clinical specimen was obtained.
Conclusions: The HCAI rate remained relatively stable over time. Routine surveillance and feedback of infection rates to the facilities did not result in a noticeable decrease of infection burden over time. It is of immediate concern that antimicrobials were being prescribed for a large proportion of suspected infections that did not meet criteria for clinical infection. Opportunities exist to further improve the use of antimicrobials in the RACF setting.