Each year, over 1000 medical device incidents are reported to the Australian Therapeutic Goods Administration (TGA). The most recent published statistical reports from this scheme show 926 reports in 6 months (January–June 2009), of which 597 resulted in serious injury and 25 were associated with patient death.1,2
The 3 Fr peripherally inserted central catheter (PICC) is a medical device commonly used in infants and children for administration of medication and fluids, and for blood sampling.3 Reported complication rates range from zero to 33.6%;4 with occlusion and catheter-related bloodstream infection being the most common. Rarer complications include phlebitis, venous or right atrial perforation and extravasation, pleural effusion, pericardial effusion or tamponade, dysrhythmias and mechanical failure, such as leakage, migration of the tip, and line fracture.4,5 Although line fracture and embolisation have been studied in children and adults,5 guide-wire fragment embolisation has been confined to a single case report.6
Devices were evaluated in a simulated clinical environment using a modified failure mode and effects analysis (FMEA) methodology.7 A “failure mode” is the way in which a device can fail.
The occurrence rating (O) is the probability that the failure mode will occur. The potential resultant harm to the patient is measured with the severity rating (S). The probability that the failure mode will actually result in harm is recorded as a likelihood rating (L). Occurrence and likelihood use the same descriptors, but measure different events (Box 1).
The risk priority number (RPN) is the product of the occurrence, severity and likelihood ratings (O × S × L), and is used to compare the level of risk associated with each of the failure modes. For each device, failure modes for each step of use were identified and RPNs calculated.
The theoretical MRI safety risk associated with heating and movement of an embolised wire fragment was evaluated.8 The ferromagnetic properties of the wires were confirmed by exposure to a magnet. A straight (7 cm) and looped (20 cm) wire fragment from each device was partially embedded in agar gel (1.5 g agar in 100 mL phosphate-buffered saline) in an 88 mm Petri dish with graph paper on the underside. Movement was detected by direct and video observations during: (1) entry into the static magnetic field of a Magnetom Trio Tim 3T MRI scanner (Siemens Healthcare, Erlangen, Germany) using syngo MR software (Siemens Healthcare); (2) 7 minutes of scanning using TrueFISP (Siemens Healthcare) (sequence trfi2d1_43, repetition time [TR] 171 ms, echo time [TE] 1.3 ms, 26 slices, 60 degree pulse angle); and (3) rotation of the plate in a static 3 T MRI field to simulate patient movement. Temperature of the wire-gel interface was measured with a thermocouple probe (Digitech thermometer, Electus Distributions, Sydney, NSW) before and after exposure to the MRI field. Potential MRI risk was identified by ferromagnetism with either movement of the wire, or a temperature rise of > 3.0°C measured next to the wire.9
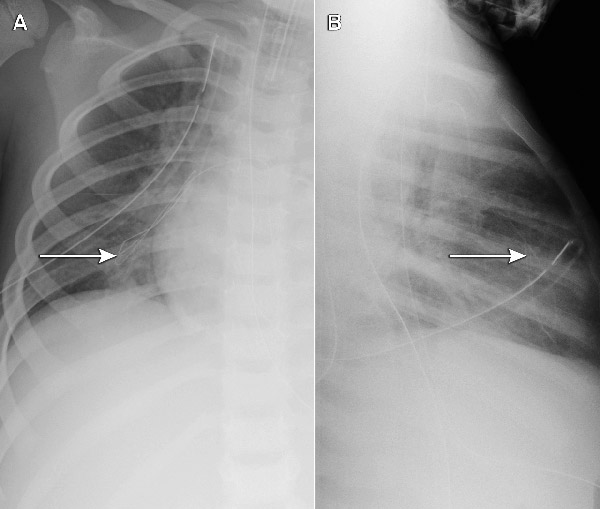
Each of the five cases identified was undetected at the time of insertion. As only part of the guide wire embolised, the portion withdrawn by the operator provided a false reassurance. The extremely fine embolised wires had not been noted on post-insertion chest x-rays (where the focus is usually on the position of the catheter tip), but were apparent when a specific review of the films was undertaken (Box 2). This cluster of five cases (involving different operators) at a single hospital triggered a statewide case review, through which an additional five cases in two further hospitals were reported (one actual embolus and four “near misses”, where the wire unravelled, but was noticed and retrieved before entering the patient). All involved the same type of device, giving a total of 10 cases at three hospitals out of 710 patients followed up. Although objective data about whether the wire was cut in every case were not available (because it can be cut without detection), at least one clinician was certain the wire had not been cut.
Initial evaluation suggested potential contributing factors including product design (a trimmable PICC with a fine-gauge wire preloaded through a constrictive septum with the potential for shearing if withdrawn), latent factors (insufficient instructions for use with inadequate warnings about not trimming the wire or withdrawing the guide wire through the septum) and human factors (eg, withdrawal of the guide wire through the septum). Only one set of instructions was available in each box of five catheters. An investigation by the TGA, which concluded in November 2008, required improvements to label warnings and user instructions, but found no inherent flaw with the PICC devices.10
The clinician survey examined user experience. Of the 55 respondents, 39 had previously inserted a 3 Fr PICC device. Information on complications, including the frequency of technical mistakes, need for multiple attempts and breakages associated with insertion, was completed by 32 respondents (Box 3). Of these, there were 23 medical practitioners, eight nurses and one sonographer.
Five practitioners (16%) had used all four devices, eight practitioners (25%) had used three devices, 10 (31%) had used two devices, and nine (28%) had used only one of the devices. No other types of PICC devices were used. Two broken-wire embolisation events were reported for the same type of device as in the original case series, but not for any of the other devices (Box 3).
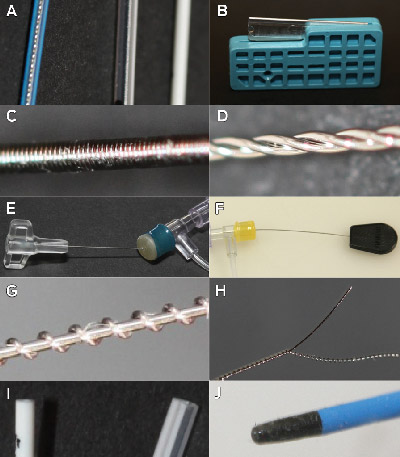
The device design evaluation assessed the latent safety features of the four devices, including design features resistant to clinician error. A range of six to 17 potential design failure modes were demonstrated across each of the four devices (Box 4). Failure modes for several clinically relevant complications were linked to five key design elements (Box 5, Box 6). The presence of all five design elements increased the risk of wire fragment embolisation.
A potential risk of an undetected wire fragment in situ was exposure to MRI resulting in tissue and thermal injury.11 All four guide wires were ferromagnetic when exposed to a magnet. On entrance into the MRI field, all wire types moved with sufficient force to pull the partially embedded wire from the agar gel and unravel the coiled wire loop. Once the plate was positioned in the centre of the MRI magnet, wire movement was not observed with application of field gradients. Gentle rotation of the plate in the centre of the scanner resulted in reorientation of all four wire types in the direction of magnetic field lines. The median temperature change in the agar gel next to the wire was 1.5°C (range, − 0.9°C to +1.8°C) with a temperature rise of 0.5°C in a control plate without a wire fragment. All devices were rated as being of potential MRI risk due to possible movement and damage to surrounding tissue.
There is no other published safety information on PICC guide wires exposed to a magnetic field. On the basis of this study, we recommend that children who have had a PICC inserted need adequate screening before MRI.
Although warning labels and product information are sufficient to achieve TGA approval, their effectiveness is weakened by their interplay with human factors. Latent design features that support safety (eg, the closed end and double-helix construction of the wire) and “forcing functions” (design features that force users down the correct usage pathway) are more reliable than complex instructions.12 Reliance on clinician vigilance and memory, in an environment of complexity or lack of standardisation, fails to acknowledge the propensity for human error.
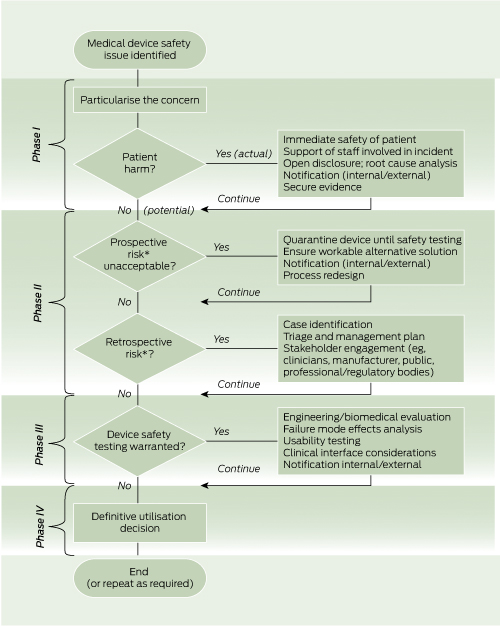
There is no standardised, published methodology for approaching this type of safety issue. Through the lessons learned in this case series and the subsequent device evaluation, we have developed a decision-making model for evaluating and managing similar safety concerns (Box 7).
1 Rating scales used in the failure mode and effects analysis
A. Occurrence and likelihood ratings |
|||||||||||||||
| B. Severity ratings | |||||||||||||||
No injury or harm caused, minor adjustment to operational routine |
|||||||||||||||
5 Failure modes and associated design features
Received 15 January 2012, accepted 14 February 2012
- Joel M Dulhunty1,2
- Andreas Suhrbier3
- Graeme A Macaulay4
- Jennifer C Brett4
- Alexa V A van Straaten1
- Ian M Brereton2
- Jillann F Farmer1,2
- 1 Patient Safety and Quality Improvement Service, Queensland Health, Brisbane, QLD.
- 2 University of Queensland, Brisbane, QLD.
- 3 Queensland Institute of Medical Research, Brisbane, QLD.
- 4 Biomedical Technology Services, Queensland Health, Brisbane, QLD.
We acknowledge the staff of the Townsville Hospital who first identified and reported this issue. We thank Dr John Wakefield for his leadership in managing the original case series in Queensland, and for his introduction of the FMEA method to clinical incident management in Queensland. Mellissa Naidoo and Kirsten Price provided assistance with obtaining ethics approval for the survey. Kevin McCaffery provided assistance with the FMEA and with obtaining ethics approval for the survey. Thank you to Joy Gardner and Wayne Schroder for laboratory assistance with the MRI safety study. Gail Durbridge and Donald Maillet helped design and conduct the MRI safety study; David Lloyd helped conduct the MRI safety study. Madeleine Kersting took photographs of the devices. Joan Curtis provided administrative support. Thank you to staff who assisted with obtaining devices for testing, participated in the survey and were involved in the notification, management and review of PICC-related incidents.
No relevant disclosures.
- 1. Therapeutics Goods Administration. Medical device incident reports 01/04/2009 – 30/06/2009. Canberra: TGA, 2009. http://www.tga. gov.au/hp/iris-articles-statistics-0904-0906.htm (accessed Feb 2012).
- 2. Therapeutics Goods Administration. Medical device incident reports 01/01/2009 – 31/03/2009. http://www.tga.gov.au/hp/iris-articles-statistics-0901-0903.htm (accessed Feb 2012).
- 3. Knue M, Doellman D, Jacobs BR. Peripherally inserted central catheters in children: a survey of practice patterns. J Infus Nurs 2006; 29: 28-33.
- 4. Pettit J. Assessment of infants with peripherally inserted central catheters: Part 1. Detecting the most frequently occurring complications. Adv Neonatal Care 2002; 2: 304-315.
- 5. Pettit J. Assessment of infants with peripherally inserted central catheters: Part 2. Detecting less frequently occurring complications. Adv Neonatal Care 2003; 3: 14-26.
- 6. Ragg P. Unravelling and embolisation of guide-wires with PICC lines, March 2007. Melbourne: Australian and New Zealand College of Anaesthetists, 2007. http://www.anzca.edu. au/quality-safety/articles/archive/unravelling-and-embolisation-of-guide-wires-with-picc-lines.html (accessed Feb 2012).
- 7. Institute for Healthcare Improvement. Failure Modes and Effects Analysis tool. Cambridge, Mass: IHI, 2011. http://www.ihi.org/knowledge/Pages/Tools/FailureModesandEffectsAnalysis Tool.aspx (accessed Feb 2012).
- 8. Shellock FG, Woods TO, Crues JV, 3rd. MR labeling information for implants and devices: explanation of terminology. Radiology 2009; 253: 26-30.
- 9. Shellock FG. The List: information and terminology. Los Angeles: MRIsafety.com, 2010. http://www.mrisafety.com/list.asp (accessed Feb 2012).
- 10. Therapeutics Goods Administration. Serious adverse events with the use of peripherally inserted central catheters (PICC lines). Canberra: TGA, 2008. http://www.tga.gov.au/safety/alerts-device-picc-081113.htm (accessed Feb 2012).
- 11. Food and Drug Administration. A primer on medical device interactions with magnetic resonance imaging systems. Silver Spring, Md: FDA, 1997. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Guidance Documents/ucm107721.htm (accessed Feb 2012).
- 12. Norman DA. The design of everyday things. New York: Basic Books, 2002.





Abstract
Objective: To report guide-wire fragment embolisation of paediatric peripherally inserted central catheter (PICC) devices and explore the safety profile of four commonly used devices.
Design, setting and participants: Clinical incidents involving paediatric PICC devices in Queensland public hospitals were reviewed. A PICC user-experience survey was conducted at five public hospitals with 32 clinicians. A device design evaluation was undertaken, and magnetic resonance imaging (MRI) safety was tested by a simulation study.
Main outcome measures: Embolisation events; technical mistakes, multiple attempts and breakages during insertion; willingness to use the device; failure modes and risk priority rating; movement and/or temperature change on exposure to MRI.
Results: Six clinical incidents of silent guide-wire embolisation, and four near misses were identified; all were associated with one type of device. The survey found that this device had a reported broken-wire embolisation rate of 0.9/100 insertions with no events in other devices; two of the four devices had a higher all-cause embolisation rate (3.3/100 insertions v 0.4/100 insertions) and lower clinician acceptance (68%–71% v 91%–100%). All devices had 6–17 identified failure modes; the two devices that allowed removal of a guide wire through a septum had the highest overall risk rating. Guide-wire exposure to MRI was rated a potential safety risk due to movement.
Conclusions: There is marked variation in the safety profile of 3 Fr PICC devices in clinical use, and safety performance can be linked to design factors. Pre-MRI screening of all children who have previously had a PICC device inserted is recommended. We advocate a decision-making model for evaluation of device safety.