Haemophilus ducreyi is a well recognised causative agent of genital ulcers and chancroid. We report two unusual cases of non-sexually transmitted H. ducreyi infection leading to chronic lower limb ulcers. Both patients were Australian expatriates visiting Australia from the Pacific Islands — one from Papua New Guinea and the other from Vanuatu.
Subsequently, a gram-negative coccobacillus was isolated on chocolate horse blood agar incubated at 35°C in CO2. The cultured organism was sent to a reference laboratory for further identification, and a presumptive diagnosis of Haemophilus ducreyi infection was made from the characteristic “schools of fish” morphology on staining (Box 1); this was confirmed with 16S rDNA (ribosomal DNA) sequencing.
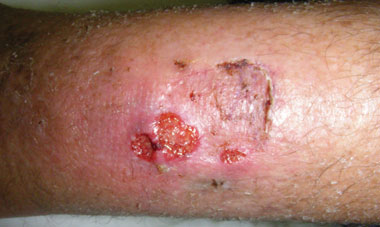
The patient was referred to an infectious diseases service. On review, the wound was 2 × 1.5 cm, with a sloughy base, rolling edges and surrounding erythema (Box 2). He had no risk factors for sexually transmissible infections and had no evidence of genital lesions or inguinal lymphadenopathy. He did not undergo testing for sexually transmitted infections. He was prescribed oral azithromycin 1000 mg, with significant improvement in the ulcer appearance. The patient returned to Papua New Guinea and was subsequently lost to follow-up.
Chronic skin ulcers secondary to H. ducreyi infection in the absence of genital lesions, as in our two cases, have not been reported until recently. H. ducreyi is a fastidious gram-negative coccobacillus. The association between this organism and chancroid, a sexually transmissible genital ulcer disease, is well established.1,2 Extragenital skin ulcers have been described resulting from autoinoculation among patients with genital ulcers.3,4 Human volunteers have developed mildly painful skin lesions following inoculation with H. ducreyi.5 Humans are the only known host for H. ducreyi; there is no recognised environmental or zoonotic reservoir.1,6
Apart from our two reported cases, to our knowledge, only two other publications have described chronic skin ulcers secondary to H. ducreyi infection.6,7 Box 3 summarises these cases and the cases we have described here. In all cases, the patients had chronic skin ulcers on lower limbs without evidence of genital ulcers or inguinal lymphadenopathy. All of these patients acquired the infection in the Pacific Islands.6,7 In most cases, the organism was cultured on selective media (eg, chocolate agar) and identified using 16S rDNA sequencing techniques.6,7
H. ducreyi is difficult to isolate from culture owing to several factors. H. ducreyi grows best between 33°C and 35°C in 5% CO2 and a humid atmosphere, and will not grow at 37°C. Enriched media, such as chocolate horse blood agar, are required for the organism’s growth. Microbiological specimens must be inoculated as quickly as possible to ensure organism survival.1 Upon Gram staining, H. ducreyi aligns in a “railroad”, “chaining” or “schools of fish” (Box 1) arrangement. Definitive identification of H. ducreyi requires specific molecular tests, such as 16S rDNA sequencing.1
Recommended treatment for H. ducreyi infection includes oral or intravenous azithromycin, ceftriaxone or ciprofloxacin.8 Plasmid-mediated β-lactamase production by this organism is well recognised; in a Californian report, 88% of H. ducreyi isolates produced plasmid-mediated β-lactamase.9 However, antimicrobial susceptibilities vary geographically. This may account for the response to penicillin or amoxicillin in three of the six cases reported thus far.
- 1. Trees DL, Morse SA. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev 1995; 8: 357-375.
- 2. Al-Tawfiq JA, Harezlak J, Katz BP, Spinola SM. Cumulative experience with Haemophilus ducreyi 35000 in the human model of experimental infection. Sex Transm Dis 2000; 27: 111-114.
- 3. Al-Tawfiq JA, Spinola SM. Haemophilus ducreyi: clinical disease and pathogenesis. Curr Opin Infect Dis 2002; 15: 43-47.
- 4. Al-Tawfiq JA, Thornton AC, Katz BP, et al. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis 1998; 178: 1684-1687.
- 5. Spinola SM, Wild LM, Apicella MA, et al. Experimental human infection with Haemophilus ducreyi. J Infect Dis 1994; 169: 1146-1150.
- 6. Ussher JE, Wilson E, Campanella S, et al. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin Infect Dis 2007; 44: e85-e87.
- 7. McBride WJ, Hannah RC, Le Cornec GM, Bletchly C. Cutaneous chancroid in a visitor from Vanuatu. Australas J Dermatol 2008; 49: 98-99.
- 8. Aldridge KE, Cammarata C, Martin DH. Comparison of the in vitro activities of various parenteral and oral antimicrobial agents against endemic Haemophilus ducreyi. Antimicrob Agents Chemother 1993; 37: 1986-1988.
- 9. Anderson B, Albritton WL, Biddle J, Johnson SR. Common beta-lactamase-specifying plasmid in Haemophilus ducreyi and Neisseria gonorrhoeae. Antimicrob Agents Chemother 1984; 25: 296-297.






We acknowledge the Microbiological Diagnostic Unit, University of Melbourne for their expertise and assistance with identification of the organism. We acknowledge Dr Gillian Wood, Medical Microbiologist and Infectious Diseases Physician, and staff at Dorevitch Pathology (Heidelberg branch), for information regarding the microbiological diagnosis of the organism in Patient 2’s case.
None identified.