Breast cancer is the most common cause of cancer-related death in Australian women, with the Australian Institute of Health and Welfare reporting 2641 deaths from the disease in 2004 and predicting 14 800 new cases in 2011.1 Population mammographic screening programs using age as the principal risk criterion have been in place in Australia since 1992. The main target population of such programs is women aged 50–69 years. In younger women at high risk of developing breast cancer, the value of mammography is limited by a higher prevalence of dense breast tissue, with low sensitivity of this imaging modality and concerns over radiation exposure.
Although up to 15% of breast cancers may have a familial component,2 it is estimated that about 5% arise from mutations in the high-risk cancer susceptibility genes BRCA13 and BRCA2.4
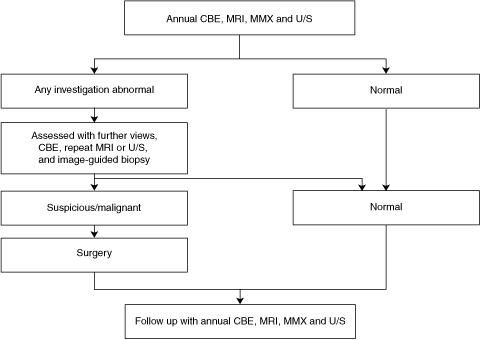
Women at high risk were advised in the National Breast and Ovarian Cancer Centre recommendations5 to have annual surveillance with a mammographic x-ray (MMX), with or without a breast ultrasound (U/S) scan and clinical breast examination (CBE), and were advised to practise breast self-examination.
The participants in our study were Western Australian women recruited from the Royal Perth Hospital’s multidisciplinary high-risk surveillance clinic. All eligible women attending over the study period were approached. Entry for our study was based on Australian risk category 3 criteria for women at potentially high risk of breast cancer due to their family history, including those with known gene mutations.5 Women at high risk due to previously diagnosed breast conditions (such as atypical ductal hyperplasia or ductal carcinoma-in-situ) were also recruited. All women were aged 50 years or under at study entry. Pregnant or lactating women and women with a personal history of invasive breast cancer were excluded.
A biopsy was performed if a lesion detected by any modality was classified as imaging category 3 or higher according to the image classification system of the American College of Radiology — the Breast Imaging Reporting and Data System (BI-RADS).6,7 This included MMX- or U/S-guided biopsies. If the lesion was visible on MRI but not on targeted U/S, short-term follow-up (at 3–6 months) was recommended. No lesions that were seen on MRI only were considered sufficiently suspicious to warrant surgical removal.
The age groups of the 72 women and their breast density categories (assessed by BI-RADS criteria)6 are summarised in Box 2.
The recall rate after MRI scans was 10.1% overall (Box 3, Box 4), declining from 9/72 cases (12.5%) in the first year to 5/67 cases (7.5%) in the second year.
No microcalcifications were visible on MMX, and nothing of significance was detected by CBE.
Numerous studies have demonstrated the value of MRI as part of imaging surveillance for women at high risk of developing breast cancer.8-13 Our study showed that MRI can be incorporated into a routine surveillance program for such women.
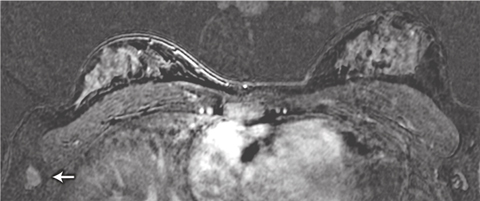
We identified two patients with lesions potentially putting them at higher risk of breast cancer and one with metastatic cancer in an axillary lymph node. The lymph node metastasis was not initially identified in the MRI scan: the patient palpated the node herself 1 month after imaging was done, and when the MRI scan was viewed retrospectively, the metastasis was in fact visible (Box 5). The patient’s imaging occurred early in the study, and failure to detect the cancer was the result of inexperience in interpreting breast MRI scans at that stage. This underlines the importance of carrying out breast MRI examination and reporting in centres that have expert multidisciplinary experience in breast surveillance, including MRI.
The BI-RADS classification system7 advises that lesions classified as category 3 on MRI should be managed by follow-up imaging. We chose to take biopsy samples of category 3 lesions because of the limited understanding of their histological correlates and the uncertain clinical significance of these lesions in women at high risk of breast cancer.
The lower recall rate following the second round of imaging (ie, in the second year of our study) can be explained by the experience gained from investigating the lesions identified in the first round, and also the availability of baseline imaging for comparison. MRI of the breast is reported to have high sensitivity but lower specificity.14 In our study, 15 lesions were detected by MRI (one of them retrospectively), but of these, 12 were false positive cases and one was a false negative. MMX and screening U/S detected fewer lesions than MRI, with only three of the 15 lesions visible on each. However, the use of targeted U/S to locate lesions seen initially only on MRI successfully identified a further six of the 15 lesions. This poses the question as to whether it is more efficient to use targeted U/S (rather than screening U/S) after MRI in patients at high risk. The low sensitivity of mammography in our study can be explained by the young age of the study population (and hence higher breast tissue density).
Given that no microcalcifications were visible on MMX in our study, the question arises as to whether high-quality one-view (lateral oblique view) mammography (rather than two-view mammography) could be used to exclude microcalcifications, thus reducing radiation exposure for young patients at high risk. This may be especially important for women with BRCA1 and BRCA2 mutations that may predispose cells to an increased risk of mutagenesis and transformation after exposure to radiation.15,16
No invasive cancers were found in our study, but two potentially high-risk lesions were detected and malignancy was excluded following excision. Four lesions were detected by MRI only and were not visible on targeted U/S. In a larger group of patients, MRI intervention tools (MR-compatible biopsy devices and MRI-guided hookwire localisations) would be necessary to sample MRI-only detected lesions17,18 and thus reduce unnecessary patient anxiety.
We would support the careful introduction of breast MRI screening in Australia within a specialist multidisciplinary setting for managing women at high risk of breast cancer. The program should include training of radiological and clinical personnel in these imaging techniques, and a detailed national audit of outcomes, as advocated by the National Working Group on Breast MRI in High Risk Women (and as detailed in the application recently approved by the Medical Services Advisory Committee), with long-term follow-up of outcomes being mandatory.19 Prospective evaluation of programs will assist in addressing the question of whether MRI screening of women at high risk of developing breast cancer may have an impact on mortality from the disease.
- Christobel M Saunders1
- Gudrun Peters1
- Glenys Longman1
- Jacqueline Thomson2
- Donna Taylor3
- Jianmin Hua3
- Michelle Bennett3
- Elizabeth Wylie1,3
- Jack Goldblatt4,5
- Arlene Chan6
- James Anderson3
- 1 School of Surgery, University of Western Australia, Perth, WA.
- 2 Perth Radiological Clinic, Perth, WA.
- 3 Royal Perth Hospital, Perth, WA.
- 4 King Edward Memorial Hospital, Perth, WA.
- 5 School of Paediatrics and Child Health, University of Western Australia, Perth, WA.
- 6 Mount Hospital, Perth, WA.
None identified.
- 1. Australian Institute of Health and Welfare and National Breast Cancer Centre. Breast cancer in Australia: an overview, 2006. Canberra: AIHW, 2006. (AIHW Cat. No. CAN 29.)
- 2. Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the Cancer and Steroid Hormone Study. Am J Hum Genet 1991; 48: 232-242.
- 3. Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 1990; 250: 1684-1689.
- 4. Wooster R, Neuhausen S, Mangion J, et al. Localisation of a breast cancer susceptibility gene BRCA2 to chromosome 13q12-13. Science 1994; 265: 2088-2090.
- 5. National Breast Cancer Centre. Advice about familial aspects of breast and ovarian cancer: a guide for health professionals. http://www.nbocc.org.au/resources/resource.php ?code=BOG (accessed Jul 2009).
- 6. BI-RADS — mammography. In: American College of Radiology. Breast Imaging Reporting And Data System (BI-RADS) Atlas. Reston, Va: ACR, 2003.
- 7. BI-RADS — magnetic resonance imaging. In: American College of Radiology. Breast Imaging Reporting And Data System (BI-RADS) Atlas. Reston, Va: ACR, 2003.
- 8. Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005; 23: 8469-8476.
- 9. Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005; 365: 1769-1778.
- 10. Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with familial or genetic predisposition. N Engl J Med 2004; 351: 427-437.
- 11. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004; 292: 1317-1325.
- 12. Podo F, Sardanelli F, Canese R, et al. The Italian multi-centre project on evaluation of MRI and other imaging modalities in early detection of breast cancer in subjects at high genetic risk. J Exp Clin Cancer Res 2002; 21 (3 Suppl): 115-124.
- 13. Stoutjesdijk MJ, Boetes C, Jager GJ, et al. Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J Natl Cancer Inst 2001; 93: 1095-1102.
- 14. Heywang-Kobrünner SH, Bick U, Bradley WG Jr, et al. International investigation of breast MRI: results of a multicentre study (11 sites) concerning diagnostic parameters for contrast-enhanced MRI based on 519 histopathologically correlated lesions. Eur Radiol 2001; 11: 531-546.
- 15. Speit G, Trenz K. Chromosomal mutagen sensitivity associated with mutations in BRCA genes. Cytogenet Genome Res 2004; 104: 325-332.
- 16. Berrington de Gonzalez A, Berg CD, et al. Estimated risk of radiation-induced breast cancer from mammographic screening for young BRCA mutation carriers. J Natl Cancer Inst 2009; 101: 205-209.
- 17. Perlet C, Heinig A, Prat X, et al. Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol 2002; 12: 1463-1470.
- 18. Viehweg P, Heinig A, Amaya B, et al. MR-guided interventional breast procedures considering vacuum biopsy in particular. Eur J Radiol 2002; 42: 32-39.
- 19. Lord SJ, Lei W, Craft P, et al. A systematic review of the effectiveness of magnetic resonance imaging as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer 2007; 43: 1905-1917.





Abstract
Objective: To determine whether a surveillance program including clinical breast examination (CBE) and three screening modalities (magnetic resonance imaging [MRI], high-resolution ultrasound [U/S] and mammographic x-ray [MMX]) was feasible, and whether it could improve detection of pathological lesions in young women at high risk of developing breast cancer.
Design, setting and participants: Western Australian women aged 50 years or under at high risk of developing breast cancer were recruited to our study. For a 2-year period, they were offered breast MRI and U/S scans in addition to their annual MMX and CBE. Our study was conducted between June 2002 and October 2005.
Main outcome measures: Number and type of cancerous or precancerous lesions; recall rates after screening; comparative sensitivity of screening modalities.
Results: Of 102 women approached, 72 agreed to participate. Fifteen lesions were detected, of which three were significant: a metastatic papillary cancer in an axillary lymph node, a borderline lesion (multiple papillomatosis with atypia), and a papilloma. All 15 lesions were visible on MRI, and four were detected by MRI only. Only one lesion was visible on all three imaging modalities. Nothing significant was detected by CBE. The recall rate after MRI scans fell from 9/72 (12.5%) in the first year to 5/67 (7.5%) in the second year.
Conclusion: Our study gave valuable experience in a team approach to screening MRI, and showed that MRI can detect more lesions than MMX or U/S in women at high risk of developing breast cancer. Screening U/S may not add value to MMX and MRI screening, and we suggest a single oblique-view MMX may be used in some cases.