Exacerbation of childhood asthma is one the most common acute presentations to general practices and emergency departments (EDs). Most of these children are not hospitalised, but asthma exacerbations are associated with significant cost1 and both acute and prolonged morbidity.2,3
The main treatments are bronchodilators and systemic corticosteroids. While corticosteroids have been shown to be effective,4-7 there is a lack of consensus regarding the dose, length and route of administration.5 There is considerable variation regarding length of administration among published guidelines8-10 and systematic reviews,4-6 vary-ing from 1–3 days to 3–10 days.6 Australian guidelines recommend oral cortico-steroids (prednisolone, 1 mg/kg, up to 60 mg daily) for up to 5 days,8 while United States guidelines recommend 1–2 mg/kg/day (in two doses) for 3–10 days.9
However, few studies have compared the dose and length of corticosteroid administration.5,11 While a longer course of oral corticosteroids might improve short-term symptoms of asthma and quality of life (QOL), adverse events seen in children after short courses of corticosteroids include behavioural changes, hallucinations, fungal infections, increased appetite and adrenal insufficiency.12,13 Thus, it is desirable to keep courses of oral corticosteroids as short as possible while retaining efficacy.
At recruitment, a clinical history and examination were undertaken and documented on a standardised data collection sheet. Questions specific to asthma (eg, number of exacerbations in previous 12 months, routine medications) were asked. Severity of acute asthma on presentation was categorised according to the Asthma Severity Scale (ASS) (0–3 = mild; ≥ 4 = moderate).14 Scores on the five-point Australasian Triage Scale (1 = immediately life-threatening; 3 = potentially life-threatening; 5 = less urgent) were also recorded.15
Baseline and weekly Paediatric Asthma Caregiver’s Quality of Life Questionnaire (PACQLQ) scores16 and validated daily diary scores for asthma17 and cough18 were recorded. The asthma score was the average of four questions.17 Follow-up phone calls occurred 24–48 hours after enrolment and at Days 7, 14, 21 and 28, where PACQLQ scores and adverse events were specifically recorded.
The main secondary outcomes were PACQLQ scores on Days 7 and 14. Other outcomes were: average asthma scores as scored on asthma17 and cough18 diary cards on Days 5, 10 and 14; recurrence of exacerbation; and unscheduled re-presentation to a health facility. In accordance with Australian guidelines,8 we did not use peak flow as an outcome measure.
The sample size required was calculated a priori from previous data.19 Assuming a dropout rate of 20%, 180 children (90 per group) were required for a reduction in proportion from 60% to 30% of children known to be symptomatic on Day 7 when given a longer course of oral corticosteroids. We recalculated the sample size for the primary outcomes after the first 30 children were enrolled, and the sample size increased to 200 for the same power. For the PACQLQ outcome, 168 children were required for a power of 90% at a 5% significance level, for a minimally important mean difference of 0.5 in PACQLQ scores.20
Data were examined for type of distribution using normality plots. We used unpaired Student’s t test for two-group comparisons of normally distributed data, the Kruskal–Wallis test for non-normal data and Pearson’s χ2 test for categorical variables. Linear regression was used to examine effect of asthma severity (ASS)14 on presentation on Day 7 symptom scores and PACQLQ scores. A two-tailed P < 0.05 was considered significant. Data were initially analysed using intention-to-treat (ITT) analysis, followed by per-protocol analysis.
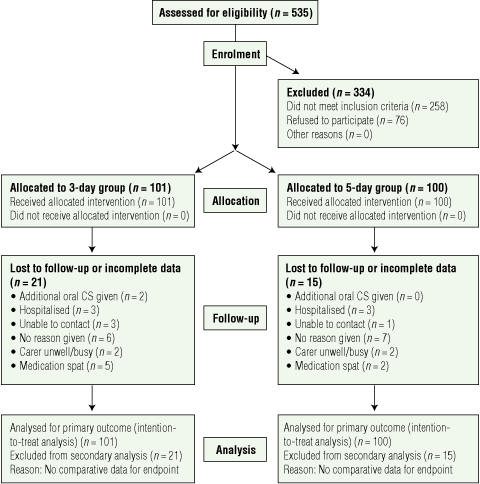
There were 201 children enrolled in the study: 175 (87%) at Royal Children’s Hospital, 17 (9%) at Mater Hospital and nine (5%) at Gympie Hospital; 36 did not complete the study (Box 1). Baseline characteristics were similar for patients in both groups and for those who completed and did not complete the study (Box 2). The reasons for dropping out for 21 children from the 3-day group and 15 from the 5-day group (P = 0.36) were also similar (Box 1).
There was no significant difference between groups in any other secondary outcome, with P values ranging from 0.17 to 0.91 (Box 3). The difference between groups in Day 5 asthma score was 0.02 (95% CI, − 0.36 to 0.40). Between Days 4 and 14, only one child (from the 3-day group) was hospitalised, and 13 received additional prednisolone (eight children from the 3-day group; five from the 5-day group). However, the difference between groups was not significant (P = 0.40).
Our results are similar to those of smaller single-centre studies, which found that a shorter duration of systemic corticosteroids was as effective as a longer course.21,22 In one study of young children (mean age, 36–37 months), the short-term morbidity (at Day 5) of 15 children who received a single dose of intramuscular dexamethasone was similar to that of 17 who received oral prednisolone for 5 days.21 However, some of these children may not have had asthma.21 In a study of 117 children aged 2–6 years, outcomes on Day 5 were similar in those given a single dose of oral dexamethasone and those given prednisolone twice daily for 5 days.22 Dexamethasone is thought to be effective for up to 72 hours,22 which amounts to a similar length of effect to 3 days of prednisolone. We used prednisolone rather than dexamethasone, as the latter is only available in hospitals and our findings would be relevant to children presenting to general practitioners with acute asthma not severe enough to require hospitalisation. However, these studies were arguably underpowered and did not examine the effect beyond the first 3–5 days.
Our study was larger and longer, and included symptom diaries and QOL measures. The importance of QOL for children with asthma has been previously highlighted,23 and outcomes beyond the period of corticosteroid administration are important, as studies have shown that morbidity beyond the immediate asthma exacerbation period is greater than expected.3 Our study thus adds important high-level evidence to the literature.
The dose of oral corticosteroids we used was similar to current guidelines, but lower than that used in previous studies.21,22 It was chosen based on current practice and evidence of adverse events when 2 mg/kg/day was used.13 Further, a systematic review found only two trials that assessed clinical responses to different doses of systemic corticosteroids; neither found a therapeutic advantage of higher doses.11
There was also no significant difference between groups when per-protocol analysis was performed (as opposed to ITT analysis). The small difference between groups in PACQLQ scores on Days 7 and 14 (0.18 and 0.17) was less than the established minimally important mean difference of 0.50 in PACQLQ scores.20 However, the upper 95% confidence limit for Day 7 PACQLQ scores (0.51) is just larger than the minimum clinically important difference, so equivalence was not shown for this outcome. The upper 95% confidence limit for PACQLQ on Day 14 was 0.44, demonstrating equivalence. Also, the difference between groups in the Day 5 asthma diary score of 0.02 is well within the non-inferiority margin of expected change in the diary score17 based on historical controls. Post-priori calculation showed that a study size of 201 patients is sufficient to be 87% sure that the upper 95% confidence limit for the difference between the two treatment groups is < 20% for symptom score on Day 4. For PACQLQ scores on Day 7, 160 children provided 99% surety that the upper 95% confidence limit for the difference between the two groups was < 0.50.
Although our study was a multicentre study, the different size of the hospitals and starting dates resulted in unequal distribution of the children enrolled. Another limitation of our study was the deviation from protocol. We had intended to include data to Day 28, as we had done in our previous study,19 but as many parents found this too difficult, our data were limited to 14 days. Nevertheless, given the negative results and the short duration of action of prednisolone, it is likely that data beyond 14 days would not have altered our findings.
Our study was unique in using patient-oriented outcomes, which are arguably as important as objective measures23 — especially in young children, in whom objective respiratory measures are limited. We also examined data beyond the immediate exacerbation period.
We conclude that a 5-day course of oral prednisolone confers no additional advantage over a 3-day course for children with an acute asthma exacerbation that does not require hospitalisation. Although we saw few adverse events in our study, more significant adverse events following short courses of oral corticosteroids may occur.12,13 Shortening courses of oral corticosteroids to the minimum effective length would limit the exposure of children to unnecessary medications and reduce costs.
2 Baseline characteristics of the children, by trial group and completion of study
3 Median scores of asthma-related morbidity in children who received a 5-day or a 3-day course of oral prednisolone
 3-day group = 3-day course of oral prednisolone plus 2-day course of placebo. 5-day group = 5-day course of prednisolone. PACQLQ = Paediatric Asthma Caregiver’s Quality of Life Questionnaire.16 |
Received 26 January 2008, accepted 6 May 2008
- Anne B Chang2
- Ronald Clark1
- Theo P Sloots3
- David G Stone4
- Helen L Petsky1
- Donna Thearle1
- Anita A Champion1
- Coralie Wheeler5
- Jason P Acworth1
- 1 Royal Children’s Hospital, Brisbane, QLD.
- 2 Menzies School of Health Research, Charles Darwin University, Darwin, NT.
- 3 Queensland Paediatric Infectious Diseases Laboratory, Sir Albert Sakzewski Virus Research Centre, Brisbane, QLD.
- 4 Department of Emergency Medicine, Mater Hospital, Brisbane, QLD.
- 5 Gympie Hospital, Gympie, QLD.
We thank the parents and children who participated in the study. We also thank the nursing and medical staff of the EDs for their support, in particular Sharon Veale, Beryl Sutton, Vivien Geldard and Carol Willis for data collection. We are also grateful to Professor Elizabeth Juniper for allowing us to use her PACQLQ without charge.
The study was supported by the Asthma Foundation of Queensland and the Royal Children’s Hospital Foundation. Anne Chang is supported by an Australian National Health and Medical Research Council Practitioner Fellowship. All of the placebo and some of the active medication were donated by Aspen Pharmacare.
None identified.
- 1. Weinberger M. Corticosteroids for exacerbations of asthma: problems and solutions. J Pediatr 2000; 136: 276-278.
- 2. Stevens MW, Gorelick MH. Short-term outcomes after acute treatment of pediatric asthma. Pediatrics 2001; 107: 1357-1362.
- 3. Benito-Fernandez J, Onis-Gonzalez E, Alvarez-Pitti J, et al. Factors associated with short-term clinical outcomes after acute treatment of asthma in a pediatric emergency department. Pediatr Pulmonol 2004; 38: 123-128.
- 4. Rowe BH, Edmonds ML, Spooner CH, Camargo CA. Evidence-based treatments for acute asthma. Respir Care 2001; 46: 1380-1390.
- 5. Smith M, Iqbal S, Elliott TM, et al. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev 2003; (1): CD002886.
- 6. Rachelefsky GS. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics 2003; 112: 382-397.
- 7. Scarfone RJ, Friedlaender E. Corticosteroids in acute asthma: past, present, and future. Pediatr Emerg Care 2003; 19: 355-361.
- 8. National Asthma Council Australia. Asthma management handbook. 2006. Melbourne: NACA, 2007.
- 9. National Heart, Lung and Blood Institute. Asthma Education and Prevention Program. Expert Panel Report 3. Guidelines for the diagnosis and management of asthma. Full report 2007. Bethesda, Md: NHLBI, 2007.
- 10. British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Revised edition 2007. London: BTC and SIGN, 2007. http://www.brit-thoracic.org.uk/ClinicalInformation/Asthma/AsthmaGuidelines/PastAsthmaGuidelines/tabid/302/Default.aspx (accessed Jul 2008).
- 11. Zhang L, Mendoza RA. Doses of systemic corticosteroids in hospitalised children with acute asthma: a systematic review. J Paediatr Child Health 2006; 42: 179-183.
- 12. Mortimer KJ, Tata LJ, Smith CJP, et al. Oral and inhaled corticosteroids and adrenal insufficiency: a case–control study. Thorax 2006; 61: 405-408.
- 13. Kayani S, Shannon DC. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: a comparison of two doses of oral steroids. Chest 2002; 122: 624-628.
- 14. Bishop J, Carlin J, Nolan T. Evaluation of the properties and reliability of a clinical severity scale for acute asthma in children. J Clin Epidemiol 1992; 45: 71-76.
- 15. Australasian College for Emergency Medicine. Guidelines on the implementation of the Australasian Triage Scale in emergency departments. Guideline 24. Melbourne: ACEM, 2005. http://www.acem.org.au/media/policies_and_guidelines/G24_Implementation__ATS.pdf (accessed Jun 2007).
- 16. Juniper EF, Guyatt GH, Feeny DH, et al. Measuring quality of life in the parents of children with asthma. Qual Life Res 1996; 5: 27-34.
- 17. Santanello NC, Barber BL, Reiss TF, et al. Measurement characteristics of two asthma symptom diary scales for use in clinical trials. Eur Respir J 1997; 10: 646-651.
- 18. Chang AB, Newman RG, Carlin J, et al. Subjective scoring of cough in children: parent-completed vs child-completed diary cards vs an objective method. Eur Respir J 1998; 11: 462-466.
- 19. Chang AB, Harrhy VA, Simpson JL, et al. Cough, airway inflammation and mild asthma exacerbation. Arch Dis Child 2002; 86: 270-275.
- 20. Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994; 47: 81-87.
- 21. Gries DM, Moffitt DR, Pulos E, Carter ER. A single dose of intramuscularly administered dexamethasone acetate is as effective as oral prednisone to treat asthma exacerbations in young children. J Pediatr 2000; 136: 298-303.
- 22. Altamimi S, Robertson G, Jastaniah W, et al. Single-dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. Pediatr Emerg Care 2006; 22: 786-793.
- 23. Juniper EF. How important is quality of life in pediatric asthma? Pediatr Pulmonol Suppl 1997; 15: 17-21.





Abstract
Objective: To determine whether a 5-day course of oral prednisolone is superior to a 3-day course in reducing the 2-week morbidity of children with asthma exacerbations who are not hospitalised.
Design, setting and participants: Double-blind randomised controlled trial of asthma outcomes following a 5-day course of oral prednisolone (1 mg/kg) compared with a 3-day course of prednisolone plus placebo for 2 days. Participants were children aged 2–15 years who presented to the emergency departments of three Queensland hospitals between March 2004 and February 2007 with an acute exacerbation of asthma, but were not hospitalised. Sample size was defined a priori for a study power of 90%.
Main outcome measures: Difference in proportion of children who were symptom-free at Day 7, as measured by intention-to-treat (ITT) and per-protocol analysis; quality of life (QOL) on Days 7 and 14.
Results: 201 children were enrolled, and there was an 82% completion rate. There was no difference between groups in the proportion of children who were symptom-free (observed difference, 0.04 [95% CI, − 0.09 to 0.18] by ITT analysis; 0.04 [95% CI, − 0.17 to 0.09] by per-protocol analysis). There was also no difference between groups in QOL (P = 0.42). The difference between groups for the primary outcome was within the equivalence range calculated post priori.
Conclusion: A 5-day course of oral prednisolone confers no advantage over a 3-day course for children with asthma exacerbations who are not hospitalised.
Trial registration: Australian Clinical Trials Registry ACTRN012605000305628.