In the mid 1990s, in response to concerns about quality of reporting of randomised controlled trials (RCTs), an international group developed the Consolidated Standards of Reporting Trials (CONSORT) statement.1,2 CONSORT is intended to improve the reporting of an RCT, enabling readers to understand its conduct and to gauge the validity of its results.
The original CONSORT statement, developed for simple two-group parallel RCTs, comprises a checklist and flow diagram.1 Presently, CONSORT includes a 22-item checklist and four-stage flow diagram.2 The items included in the checklist were selected, whenever possible, because empirical evidence indicates that not reporting the information is associated with biased estimates of treatment effectiveness, or because the information is essential to judge the reliability or relevance of the findings.2-6 CONSORT has been endorsed by prominent medical journals and by editorial groups, such as the International Committee of Medical Journal Editors.
Since the publication of CONSORT, several evaluations of its effectiveness have been published.7-10 Two articles have suggested that journal endorsement of CONSORT is associated with improved quality of reporting.7,8 However, other study results have been equivocal.9,10 To gain a broader global perspective of CONSORT’s effectiveness, we conducted a systematic review, examining effectiveness in journals that have formally endorsed CONSORT.
Studies were eligible if they were comparative studies evaluating the quality of RCT reporting; one of the comparator groups included RCTs reported in CONSORT-adopting journals; and outcomes reported included any of the 22 items on the CONSORT checklist, summary scores based on the CONSORT checklist, or any measures of overall trial quality.11 Study quality was not an exclusion criterion, and studies were not excluded based on publication status or language of publication.
A single non-blinded reviewer (A C P) assessed reporting quality using a five-item checklist based on principles of internal and external validity.12 Quality assessment included the use of a control group, RCT cohort ascertainment, how the authors reached agreement on whether a checklist item was included, and whether the reviewers were blinded when assessing outcomes.
Our search strategy identified 1128 studies; 248 were potentially relevant and eight of them7,8,13-18 met our inclusion criteria (Box 1). One study was included after the author provided unpublished data.17 Six studies that examined changes in the quality of trial reporting over time or compared quality of trial reporting between journals were excluded, either because they did not include a comparator group or were not published in CONSORT-adopting journals,9,19-21 or because insufficient detail was available to determine eligibility.10,22
The number of RCTs in individual studies ranged from 13 to 240, and the number of journals in individual studies ranged from 1 to 68 (Box 2). All studies were published in English. One study used a different definition of CONSORT adoption (journals in which the editor applied the CONSORT checklist were considered CONSORT adopters),7 but 88% of RCTs in the study met our definition of CONSORT adoption. For all other included studies, the definition of CONSORT adopters matched our definition.
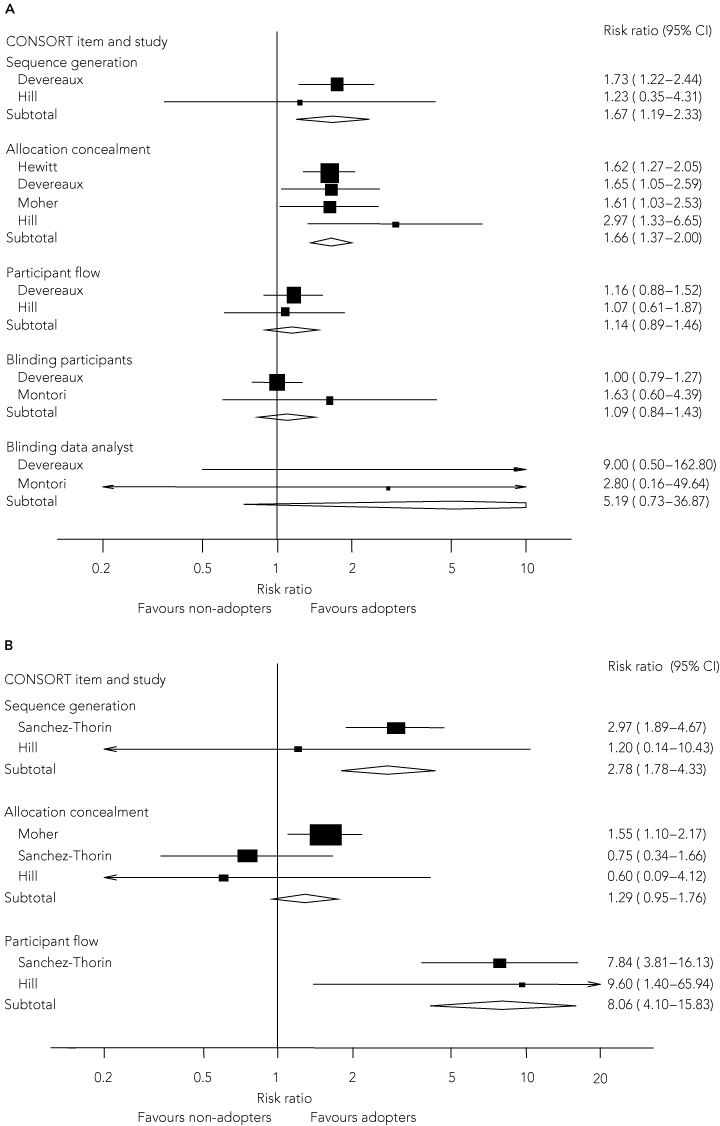
Overall, 37 different outcomes were reported (range, 1–16 per study) (Box 3). This variability resulted in most outcomes being reported in only one study. Outcomes used in more than one study were reporting of allocation concealment (five studies), sequence generation (three studies), blinding (four studies), participant flow (two studies) and overall number of CONSORT checklist items (two studies, one using a total of 30 items, the other 40 items). Blinding as an outcome was assessed as reported or not in one study,17 and blinding of participants and data analysts in three studies.7,17,18
Box 2 summarises the methodological quality of the studies. All studies were quasi-experimental, with only one study having an a priori defined control group.8
CONSORT-adopting journals had better reporting of the method of sequence generation and allocation concealment (Box 4A). For the two studies that reported total number of CONSORT items, the standardised mean difference was 0.83 (95% CI, 0.46–1.19). CONSORT adoption appeared to have less effect on reporting of participant flow and reporting of blinding of participants or data analysts.
The descriptions of the method of sequence generation, participant flow and total CONSORT items (standardised mean difference, 3.67 items; 95% CI, 2.09–5.25) were better after adoption of CONSORT (Box 4B). CONSORT adoption appeared to have less effect on allocation concealment. The paucity of data precluded meaningful examination of publication bias. There was no evidence of heterogeneity.
Our results are encouraging, but provide no definitive answer as to whether the CONSORT checklist improves reporting of RCTs. Perhaps unique to our review is ascertaining whether the RCTs were published in journals that adhered to CONSORT. If journals do not enforce the use of the checklist, it is likely that the effects we observed underestimate the possible benefits with proper enforcement of CONSORT. A review of 167 high-impact journals found that only 22% mentioned CONSORT in their instructions to authors, and 25% of these referred to the obsolete 1996 version.24 In another study involving 15 high-impact journals (five of which were not included in the previously mentioned review) that have reported endorsing CONSORT, only eight referred to the statement in their instructions to authors.25 CONSORT-adopting journals should be more proactive in enforcing adherence to CONSORT.
Although we did not find a strong influence of CONSORT on the quality of reporting of blinding, a recent study has suggested that the CONSORT checklist may have had a positive influence. In a study of RCTs in journals that adopted the original and then the revised CONSORT checklist, the authors found that the quality of reporting of blinding improved by 23%–55% between publication of the two checklists.26 This is particularly important in light of emerging data suggesting investigators, educators and readers vary greatly in their interpretations and definitions of types of blinding.27
2 Characteristics and quality of included studies
Jefferson Smurfit Foundation, Ireland; US National Library of Medicine; Merck; Glaxo Wellcome; CIHR |
|||||||||||||||
Received 7 December 2005, accepted 22 May 2006
- Amy C Plint1
- David Moher1,2
- Andra Morrison3
- Kenneth Schulz4
- Douglas G Altman5
- Catherine Hill6
- Isabelle Gaboury2
- 1 University of Ottawa, Ottawa, Ontario, Canada.
- 2 Chalmers Research Group, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada.
- 3 Canadian Coordinating Office for Health Technology Assessment, Ottawa, Ontario, Canada.
- 4 Quantitative Sciences, Family Health International, Durham, NC, USA.
- 5 Centre for Statistics in Medicine, Oxford, UK.
- 6 Rheumatology Unit, Queen Elizabeth Hospital, Adelaide, SA.
None identified.
- 1. Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA 1996; 276: 637-639.
- 2. Moher D, Schulz KF, Altman D for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001; 285: 1987-1991.
- 3. Hollis S, Campbell F. What is meant by intention-to-treat analysis? Survey of published randomized controlled trials. BMJ 1999; 319: 670-674.
- 4. Ruiz-Canela M, Martinez-Gonzalez MA, de Irala-Estevez J. Intention to treat analysis is related to methodological quality [letter]. BMJ 2000; 320: 1007-1008.
- 5. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995; 273: 408-412.
- 6. Moher D, Pham B, Jones A, et al. Does the quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998; 352: 609-613.
- 7. Devereaux PJ, Manns BJ, Ghali WA, et al. The reporting of methodological factors in randomized controlled trials and the association with a journal policy to promote adherence to the Consolidated Standards of Reporting Trials (CONSORT) checklist. Control Clin Trials 2002; 23: 380-388.
- 8. Moher D, Jones A, Lepage L for the CONSORT Group. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA 2001; 285: 1992-1995.
- 9. Piggot M, McGee H, Feuer D. Has CONSORT improved the reporting of randomized controlled trials in the palliative care literature? A systematic review. Palliat Med 2004; 18: 32-38.
- 10. Bhandari M, Guyaa G, Lochner H, et al. Application of the Consolidated Standards of Reporting Trials (CONSORT) in the fracture care literature. J Bone Joint Surg Am 2002; 84: 485-489.
- 11. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1-12.
- 12. Campbell DC, Stanley JT. Experimental and quasi-experimental design for research. Boston: Houghton Mifflin Company, 1966.
- 13. Hewitt C, Hahn S, Torgerson DJ, et al. Adequacy and reporting of allocation concealment: a review of recent trials published in four general medical journals. BMJ 2005; 330: 1057-1058.
- 14. Halpern SH, Darani R, Douglas MJ, et al. Compliance with the CONSORT checklist in obstetric anaesthesia randomised controlled trials. Int J Obstet Anesth 2004; 13: 207-214.
- 15. Faunce TA, Buckley NA. Of consents and CONSORTS: reporting ethics, law, and human rights in RCTs involving monitored overdose of healthy volunteers pre and post the “CONSORT” guidelines. J Toxicol Clin Toxicol 2003; 41: 93-99.
- 16. Montori VM, Bhandari M, Devereaux PJ, et al. In the dark: the reporting of blinding status in randomized controlled trials. J Clin Epidemiol 2002; 55: 787-790.
- 17. Hill CL, LaValley MP, Felson DT. Secular changes in the quality of published randomized clinical trials in rheumatology. Arthritis Rheum 2002; 46: 779-784.
- 18. Sanchez-Thorin JC, Cortes MC, Montenegro M, Villate N. The quality of reporting of randomized clinical trials published in Ophthalmology. Ophthalmology 2001; 108: 410-415.
- 19. Latronico N, Botteri M, Minelli C, et al. Quality of reporting of randomised controlled trials in the intensive care literature: a systematic analysis of papers published in Intensive Care Medicine over 26 years. Intensive Care Med 2002; 28: 1316-1323.
- 20. Doig GS, Simpson F, Delaney A. A review of the true methodological quality of nutritional support trials conducted in the critically ill: time for improvement. Anesth Analg 2005; 100: 527-533.
- 21. Torgerson CJ, Torgerson DJ, Birks YF, Porthouse J. A comparison of randomised trials in health and education. Br Educ Res J 2005; 31: 761-785.
- 22. Stinson JN, McGrath PJ, Yamada JT. Clinical trials in the Journal of Pediatric Psychology: applying the CONSORT statement. J Pediatr Psychol 2003; 28: 159-167.
- 23. Scherer RW, Crawley B. Reporting of randomized clinical trial descriptors and use of structured abstracts. JAMA 1998; 280: 269-272. Correction in JAMA 1998; 280: 1054.
- 24. Altman DG. Endorsement of the CONSORT statement by high impact medical journals: a survey of instructions for authors. BMJ 2005; 330: 1056-1057.
- 25. Mills E, Wu P, Gagnier J, et al. An analysis of general medical and specialist journals that endorse CONSORT found that reporting was not enforced consistently. J Clin Epidemiol 2005; 58: 662-667.
- 26. Mills EJ, Wu P, Gagnier J, et al. The quality of randomized trial reporting in leading medical journals since the revised CONSORT statement. Contemp Clin Trials 2005; 26: 480-487.
- 27. Devereaux PJ, Manns BJ, Ghali WA, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA 2001; 285: 2000-2003.






Abstract
Objective: To determine whether the adoption of the CONSORT checklist is associated with improvement in the quality of reporting of randomised controlled trials (RCTs).
Data sources: MEDLINE, EMBASE, Cochrane CENTRAL, and reference lists of included studies and of experts were searched to identify eligible studies published between 1996 and 2005.
Study selection: Studies were eligible if they (a) compared CONSORT-adopting and non-adopting journals after the publication of CONSORT, (b) compared CONSORT adopters before and after publication of CONSORT, or (c) a combination of (a) and (b). Outcomes examined included reports for any of the 22 items on the CONSORT checklist or overall trial quality.
Data synthesis: 1128 studies were retrieved, of which 248 were considered possibly relevant. Eight studies were included in the review. CONSORT adopters had significantly better reporting of the method of sequence generation (risk ratio [RR], 1.67; 95% CI, 1.19–2.33), allocation concealment (RR, 1.66; 95% CI, 1.37–2.00) and overall number of CONSORT items than non-adopters (standardised mean difference, 0.83; 95% CI, 0.46–1.19). CONSORT adoption had less effect on reporting of participant flow (RR, 1.14; 95% CI, 0.89–1.46) and blinding of participants (RR, 1.09; 95% CI, 0.84–1.43) or data analysts (RR, 5.44; 95% CI, 0.73–36.87). In studies examining CONSORT-adopting journals before and after the publication of CONSORT, description of the method of sequence generation (RR, 2.78; 95% CI, 1.78–4.33), participant flow (RR, 8.06; 95% CI, 4.10–15.83), and total CONSORT items (standardised mean difference, 3.67 items; 95% CI, 2.09–5.25) were improved after adoption of CONSORT by the journal.
Conclusions: Journal adoption of CONSORT is associated with improved reporting of RCTs.