Multiple injections of insulin are the mainstay of treatment for type 1 diabetes mellitus and, despite improvements in insulin administration and monitoring, long-term complications remain a problem for many patients. Between 30% and 60% of patients have evidence of end-organ complications within 15 years of their initial diagnosis.1-3
The concept that transplanting the islets of Langerhans would provide superior diabetic control was first proposed over 40 years ago. In 1966, Kelly et al4 showed that normoglycaemia without exogenous insulin administration could be achieved by whole-organ vascularised pancreas transplantation. Further proof of the principle was demonstrated by Lacy and colleagues, who showed that transplantation of isolated islets could control streptozocin-induced diabetes in rodents.5,6 However, reproducible, reliable and long-lasting diabetic control with islet transplantation has been difficult to achieve in humans.7 More recently, a substantial improvement in the success rate of islet transplantation was achieved by Shapiro and colleagues.8 Their success was based on principles that included selection of appropriate patients for transplantation, use of a non-toxic effective immunosuppressive regimen, isolation of appropriate numbers of viable islets, and transplantation of sufficient numbers of islets to control blood glucose levels. Their endeavours have stimulated renewed interest in islet transplantation as a therapy for a select group of patients in whom the risks of immunosuppression are considered less than the risks of continued dependency on insulin therapy. The patients most likely to fulfil these criteria are a small group of patients who, because of defective hormonal counter-regulation and/or autonomic neuropathy, develop life-threatening hypoglycaemia without the usual warning symptoms. These patients are at risk of undetected life-threatening coma unless a third party is present when coma occurs.
Islets were separated by the closed loop method described by Ricordi et al.9,10 Pancreases were removed from heart-beating deceased donors. The pancreas was disaggregated by infusing the ducts with cold Liberase enzyme (Liberase Human Islet, Roche Applied Science, Indianapolis, Ind, USA). Dissociated islet and acinar tissue was separated on a continuous Biocoll (Biochrom AG, Berlin) density gradient (polysucrose 400 and amidotrizoic acid) on a refrigerated apheresis system (Model 2991, COBE Laboratories, Lakewood, Colo, USA).
Purified islets were counted and islet number and mass were expressed in terms of islet equivalents (IEQ).11 Islet preparations underwent a pre-transplant quality assurance process, which included a gram stain, purity and viability assessment, packed cell volume measurement and evaluation of islet morphology to exclude excessive fragmentation. Aliquots of transplanted islet preparations underwent microbiological culture, endotoxin assay and calculation of a glucose stimulation index (based on an in-vitro test of response to a glucose stimulus).12
Of 50 patients referred for evaluation by an endocrinologist, six were selected for our trial. Patient characteristics and graft outcomes are shown in Box 1. All had undetectable C-peptide levels before transplant and all had evidence of severe hypoglycaemia unawareness that had failed to respond to intense management of their insulin therapy. In addition to hypoglycaemia unawareness, one patient had antibodies to exogenous insulin and required immunosuppression with azathioprine to maintain adequate glycaemic control. Apart from hypoglycaemia unawareness, the patients were remarkably free of end-organ diabetic complications. Mean GFR was 132 mL/min/1.73m2 (range, 104–153 mL/min/1.73m2). Two had had laserphotocoagulation treatment for diabetic retinopathy, two had mild subclinical neuropathy, and two had hypertension (with blood pressure well controlled by an angiotensin-converting enzyme [ACE] inhibitor) and microalbuminuria (maximum protein excretion when not taking an ACE inhibitor was 257 mg/day but normal when taking medication).
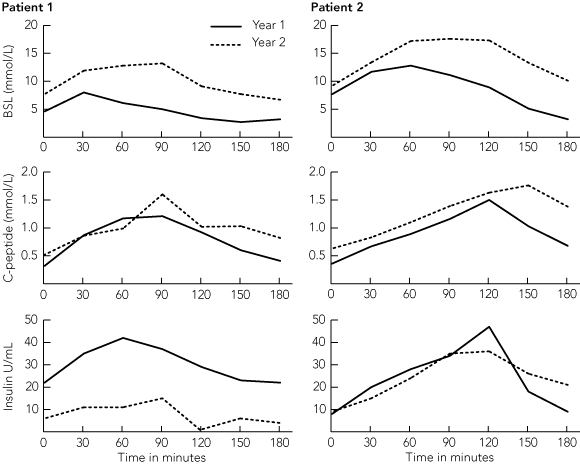
The six selected patients underwent islet transplantation between October 2002 and February 2005. The median follow-up time was 18 months (range, 3–31 months). The mean number of islet equivalents transplanted was 17 958 IEQ/kg (range, 6995–26 480 IEQ/kg). The absolute number of islets transplanted did not correlate with success in achieving insulin independence (Box 2). Five of the six patients received two islet infusions. One patient developed a portal vein thrombosis after the first infusion, had no measurable C-peptide (indicating there was no graft function), and was withdrawn from the study. Of the five remaining patients, all had evidence of islet function after the first graft. C-peptide levels and total insulin dose before and after transplant are summarised in Box 3. Three recipients were able to cease insulin treatment completely for a period of time. However, one of them withdrew from the study after 7.5 months because of intolerance to the immunosuppressive medication, suffering nausea and mouth ulcers. The remaining two had evidence of substantial islet function, with evidence of C-peptide secretion and major reductions in insulin dose.
All recipients with evidence of islet function showed marked improvements in blood glucose control and haemoglobin A1c (HbA1c) level (Box 3). In all patients, there was a reduction in insulin requirement and a fall in HbA1c level after the first islet infusion. There was a further fall in insulin requirement after the second infusion, even in recipients who did not completely stop taking insulin. Severe hypoglycaemic episodes were abolished. Three patients had episodes of mild hypoglycaemia (blood sugar level, 2.5 –3.5 mmol/L), but none required third-party intervention. These episodes became less frequent or ceased with time and were rarely seen 6 months after the second transplant. In the two patients (Patients 1 and 2) who received a second islet transplant and were insulin free for 2 years, there was evidence of loss of islet function with impairment of glucose tolerance towards the end of the second year (Box 4). Both patients have now returned to insulin therapy. Patient 1 has good control with 6–10 U insulin a day, and remains C-peptide positive with HbA1c level < 7%. (Outcomes were the same for Patient 2 until the graft ultimately failed.) Episodes of mild hypoglycaemia did occur in Patient 1 following the onset of graft dysfunction, but none of these required intervention by a third party. In Patient 3, who ceased immunosuppressive treatment after graft loss, and Patient 5, who never achieved any graft function, episodes of severe hypoglycaemia continued to occur.
However, beyond 12 months, there was evidence of a progressive loss of β-cell function. Both patients with long-term insulin independence had to return to taking a small dose of exogenous insulin, even though there was evidence of ongoing islet function and prevention of hypoglycaemia. This was consistent with previously published reports showing chronic graft loss and a reduction in β-cell secretory responses at 12 months.13,14
Our findings are consistent with similar North American and European studies.8,14-17 In our study, 80% of patients achieved graft function, with 33% achieving insulin independence for 2 years. The Edmonton group’s most recent published results of 65 recipients showed that 44 patients (68%) achieved insulin independence initially, but only 38% of those remained insulin-independent at 2 years.18
1 Summary of patient characteristics and graft outcome
Antibodies to exogenous insulin detected; recurrence of tuberculosis; failed graft |
|||||||||||||||
GFR = glomerular filtration rate. na = not applicable. tx = transplant. |
|||||||||||||||
Received 26 August 2005, accepted 12 January 2006
- Philip J O’Connell1
- Wayne J Hawthorne2
- Brian J Nankivell3
- Anita T Patel4
- Stacey N Walters5
- Henry C C Pleass6
- Richard D M Allen7
- Jeremy R Chapman8
- D Jane Holmes-Walker9
- Jenny E Gunton10
- 1 National Pancreas Transplant Unit, University of Sydney at Westmead Hospital, Westmead, NSW.
- 2 Department of Endocrinology, Westmead Hospital, Westmead, NSW.
Our study was supported by grants from the Celia Kilkeary Foundation, the National Health and Medical Research Council of Australia and the Juvenile Diabetes Research Foundation. Sirolimus was provided without charge by Wyeth Australia Pty Ltd.
Wyeth Australia provided the sirolimus for 1 year for each patient without charge. Wyeth had no role in the design, data collection, analysis, interpretation, writing or publication of this article.
- 1. US Department of Health and Human Services. National diabetes statistics fact sheet: general information and national estimates on diabetes in the United States, 2003. Bethesda: Centers for Disease Control and Prevention, 2004.
- 2. Weis U, Turner B, Watts GF, et al. Long-term predictors of coronary artery disease and mortality in type 1 diabetes. QJM 2001; 94: 623-630.
- 3. McCarty DJ, Fu CL, Harper CA, et al. Five-year incidence of diabetic retinopathy in the Melbourne Visual Impairment Project. Clin Experiment Ophthalmol 2003; 31: 397-402.
- 4. Kelly WD, Lillehei RC, Merkel FK, et al. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61: 827-837.
- 5. Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery 1972; 72: 175-186.
- 6. Kemp CB, Knight MJ, Scharp DW, et al. Transplantation of isolated pancreatic islets into the portal vein of diabetic rats. Nature 1973; 244: 447.
- 7. Brendel M, Hering B, Schulz A, Bretzel R. International Islet Transplant Registry report. Giessen, Germany: University of Giessen, 1999.
- 8. Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type I diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230-238.
- 9. Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38 Suppl 1: 140-142.
- 10. Linetsky E, Bottino R, Lehman R, et al. Improved human islet isolation using a new enzyme blend, Liberase. Diabetes 1997; 46: 1120-1123.
- 11. Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat 1990; 27: 185-195.
- 12. Lakey JR, Warnock GL, Brierton M, et al. Development of an automated computer-controlled islet isolation system. Cell Transplant 1997; 6: 47-57.
- 13. Rickels MR, Schutta MH, Markmann JF, et al. Beta-cell function following human islet transplantation for type 1 diabetes. Diabetes 2005; 54: 100-106.
- 14. Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 2005; 5: 2037-2046.
- 15. Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 2001; 50: 710-719.
- 16. Fiorina P, Gremizzi C, Maffi P, et al. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care 2005; 28: 1358-1365.
- 17. Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4: 390-401.
- 18. Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54: 2060-2069.





Abstract
Objective: To determine whether pancreatic islet transplantation can control diabetes and prevent severe life-threatening hypoglycaemia.
Design, setting and participants: A single-arm observation study of six patients undergoing islet transplantation. All patients had had type 1 diabetes mellitus for over 5 years and documented episodes of repeated severe hypoglycaemia. Islets were isolated from donor pancreases digested by Liberase. Separated islets were infused into the recipient’s liver via the portal vein. Patients were immunosuppressed with daclizumab, sirolimus and tacrolimus. The transplants were performed at Westmead Hospital, NSW, between October 2002 and February 2005.
Main outcome measures: Normal blood glucose control without administration of exogenous insulin; demonstration of islet function and abolition of hypoglycaemia.
Results: Five of the patients received two islet infusions, and the sixth was withdrawn after one infusion following a portal vein thrombosis. Three patients became insulin-independent, with excellent glycaemic control. Two had islet function with circulating C-peptide, improved glycaemic control, reduced insulin requirement and abolition of severe hypoglycaemia. However, over a 2-year period, graft function deteriorated. Recipients who were initially insulin free remained C-peptide positive but required supplemental insulin. Complications included one postoperative bleed, two portal vein thromboses (which resolved completely), presumed recurrence of tuberculosis in one patient, and deterioration in renal function in one patient.
Conclusions: Islet transplantation is effective at improving glycaemic control and hypoglycaemia unawareness in the short to medium term. However, problems with long-term safety of immunosuppression, islet-induced thrombosis and early detection of loss of islet function remain to be addressed.