The incidence of acute lower respiratory tract infection (ALRI) in the Indigenous populations of affluent countries, such as Australia and the United States,1 is similar to the incidence in developing countries. The Northern Territory has the highest proportion of Indigenous Australians of all Australian states and territ-ories, and respiratory disease is the NT’s leading cause of hospitalisation.1 In central Australia, hospitalisation of a child usually entails evacuation to the regional hospital in Alice Springs. This causes social dislocation as well as a major financial cost in an impoverished population. There is also evidence in this population that the number of episodes of hospitalisation for ALRI is a risk factor for later respiratory morbidity and bronchiectasis.2 An intervention that reduces ALRI severity or frequency of admissions for ALRI would be beneficial.
In developing countries, zinc and vitamin A supplementation has been studied for the prevention and treatment of ALRI and dia-rrhoea. Supplements of these micronutrients have been shown to reduce ALRI rates and to hasten clinical recovery from clinically defined pneumonia.3,4 While supplementation with vitamin A has been shown to reduce childhood mortality in some diseases,5 a recent meta-analysis showed no benefit on clinical recovery from pneumonia.6 However, many of the effects of vitamin A depend on zinc,7,8 providing a basis for combined use of these micronutrients.
The micronutrient status of Indigenous Australian children in remote areas is unknown. Although there is no clinical evidence of micronutrient deficiency, malnutrition is a common problem.1 Thus, it was postulated that micronutrient supplementation would benefit these children. Indeed, at the time the study was performed — when meta-analysis results6 were not yet available — some Indigenous children hospitalised with diarrhoea or pneumonia were being treated with vitamin A and zinc supplements.
Because of the risk of increased morbidity with these micronutrients (eg, anaemia, neutropenia)9,10 and variation in study results,4,9 we considered that a clinical trial was needed to justify clinical policy on supplementation.
We conducted a hospital-based randomised controlled study to examine the effect of zinc and vitamin A supplementation in Indigen-ous children hospitalised for ALRI on time to clinical recovery and incidence of readmission for ALRI during the subsequent 4 months. This was part of a larger study of micronutrient supplementation in Indigenous children with diarrhoea or pneumonia.11
A randomised controlled, 2-by-2 factorial trial of supplementation with zinc and vitamin A was conducted at Alice Springs Hospital, as previously described.11 Briefly, all Indigenous (defined by carers) children aged under 11 years who were admitted to the hospital with ALRI between April 2001 and July 2002 were eligible.
An episode of ALRI was defined as:
an illness with tachypnoea and either documented fever (temperature ≥ 38.5°C) or chest indrawing; or
pneumonia diagnosed by chest x-ray.
Tachypnoea was defined as respiratory rate at or above 60 breaths per min (age < 2 months), 50 per min (2–11 months), or 40 per min (12–59 months),12 or an arbitrary cut-off of 30 per min at age 5 years and over.
Exclusion criteria were presence of bronchiolitis syndrome (wheezing and coryza), established chronic lung disease (eg, bronchiectasis, chronic cough for over 2 months), established gastrointestinal disease (eg, short gut syndrome), or neurological disease.
Written consent to participate was obtained from the carer, and the study was approved by the Central Australia Ethics Committee and the Queensland Institute of Medical Research Ethics Committee.
Patients were randomised by computer-generated permutated block design, stratified by age (< 12 months and ≥ 12 months), to receive one of four treatments (zinc supplement plus vitamin A supplement; zinc supplement plus vitamin A placebo, zinc placebo plus vitamin A supplement, or zinc placebo plus vitamin A placebo).11 Allocation was concealed from the local research team, children, carers, and nurses who recorded daily data. However, as active and placebo vitamin A differed in appearance, and active and placebo zinc differed in taste, the allocation of vitamin A and possibly zinc was known to the team member who administered the medication.
Trial medications were commenced within 24 hours of admission and were administered by a member of the research team while the child remained in hospital. Vitamin A (50 000 IU at age < 12 months; 100 000 IU at age ≥ 12 months) was administered on Days 1 and 5, and zinc sulfate (20 mg at age < 12 months; 40 mg at ≥ 12 months) was administered daily for 5 days.
Baseline data were collected before trial medications were begun. Temperature was measured per axilla using a digital thermometer. All children had a chest x-ray interpreted by the treating paediatrician.
Serum levels of zinc, vitamin A, retinol-binding protein and albumin were measured when possible (ie, in enrolled children who had an intravenous cannula inserted as part of treatment after they were enrolled, as we could not ethically perform venepuncture for research purposes).
Primary outcome was hospital readmission for ALRI within 120 days of Day 1 of the index admission. Secondary outcomes were duration of hospital stay, oxygen saturation measured by pulse oximetry (Spo2) in air, and resolution of fever (temperature < 37.5°C) and tachypnoea. Children were followed up for 120 days for readmission for ALRI. Children who were readmitted more than 120 days after the first admission date were eligible to re-enrol and were randomly allocated to a new treatment regimen (Box 1).
The study included 215 episodes of ALRI in 187 children (Box 1). Twenty-eight children were readmitted with ALRI more than 120 days after the first admission, and were re-enrolled. The overall participation rate (including the diarrhoea arm of the study) was 96.6%. The four treatment groups had similar baseline characteristics (Box 2). Tachypnoea and fever were present in less than half the episodes, but radiological evidence of lobar pneumonia was present in 92%.
Most children who failed to complete the allocated intervention did so because they were discharged before Day 5 of the admission. For vitamin A, 66% of the supplement group and 65% of the placebo group received two doses (P = 0.58). For zinc, 95% of the supplement group and 96% of the placebo group received four or more doses (P = 0.81). Most (85%) of those assigned to receive zinc took the drug, while 13 (12%) spat some out, two took a small amount, and one vomited. Corresponding rates in the zinc placebo group were 2% each for spitting out and vomiting. All but two of those assigned to vitamin A took the drug (one was prescribed “nil by mouth” and one had no information recorded). There were no drop-outs or withdrawals. No significant adverse events were recorded.
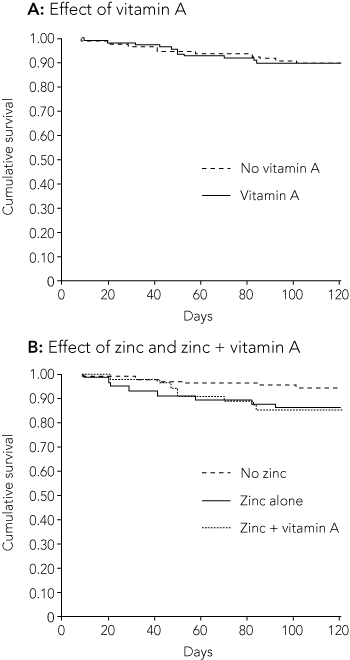
In 22 of the 215 ALRI episodes (10%), the child was readmitted with ALRI within 120 days of the previous admission: 8 after receiving zinc alone; 8, both zinc and vitamin A; 3, vitamin A alone; and 3, placebo.
Neither vitamin A nor zinc supplementation had any beneficial outcomes (Box 3). Indeed, the zinc-supplemented group were almost 2.5 times more likely to be readmitted within 120 days than the placebo group (Boxes 3 and 4). However, the latter difference was only just significant. When data were restricted to the first admission only (n = 187), the relative risk was similar, at 2.5 (95% CI, 1.03–6.43).
The interaction term zinc–vitamin A supplementation was not statistically significant (Box 3) and therefore was not included in the final model.
Analysis based on children’s nutritional status (wasted, stunted or underweight) found no difference between groups. However, results were based on small numbers (not shown). The decrease in hospital stay for underweight children in the zinc-supplemented group (median, 5 days; range, 2–9 days) when compared with the relevant placebo group (median, 9 days; range, 2–25 days; P = 0.06) was just outside the significance level.
In this randomised controlled trial we found no beneficial effect of vitamin A and/or zinc supplementation in Indigenous children hospitalised with ALRI. Instead, we found that children given zinc supplements had increased risk of readmission with ALRI.
While many trials have been published on the effect of zinc and vitamin A supplements on common childhood illnesses in developing countries,4,13 there are no published data on their effect in Indigenous children living in developed countries, who also experience malnutrition and poor living conditions. In developing countries, zinc supplementation has generally had positive effects for treatment or prevention of ALRI, and a meta-analysis found that it reduced the relative risk of pneumonia in children.4 However, these studies were mostly in areas where zinc deficiency is prevalent.14 In contrast, we found that zinc supplementation had no significant impact on respiratory morbidity and significantly increased the relative risk of subsequent ALRI requiring hospitalisation. Similarly, an increased incidence of ALRI was found in Bangladeshi9 and rural Guatemalan15 children receiving zinc.
A possible explanation for our findings is that our study population is not zinc deficient, as severe malnutrition is not as prevalent as in developing countries. Zinc improves immune function in zinc deficiency,14,16 but excessive zinc may increase infections10 by interfering with the action of copper17 and increasing susceptibility to certain microorganisms.18 Hence, it might delay pulmonary healing or enhance pulmonary injury.19
There are also conflicting reports on the effect of large doses of vitamin A for both treatment and prevention of disease.20 Malnourished Vietnamese children who received large doses of vitamin A had shorter recovery times from pneumonia.21 However, large doses of vitamin A were found to have no benefit in a study of Brazilian children with clinically diagnosed pneumonia,22 and in a recent meta-analysis of five studies in children with non-measles-related ALRI in developing countries.6 Poss-ibly, the effects of vitamin A on the immune system differ between vitamin A-sufficient and deficient children.20
Our findings should be considered in the context of the study methods. Participants were eligible to re-enrol if they were rehospitalised more than 120 days after the first admission. However, we used a statistical method to adjust for this, and results were similar when the analysis was limited to the first admission only. Our high response rate (97%) limits the potential for selection bias. Observation bias might have affected results, as members of the research team directly involved in the study were not blinded to vitamin A supplementation. However, as treatment allocation was concealed from nurses collecting data, children and carers, this bias is unlikely. Increased severity of ALRI in one group could potentially influence results, but severity (as determined by tachypnoea and pulse oximetry) was not statistically different between groups.
In addition, the study had little information on baseline serum levels of zinc and vitamin A. If the supplement groups had higher baseline micronutrient levels than the placebo groups, then results could have been biased to a nil effect. However, in the small percentage of childen tested, there was no significant difference in baseline serum levels between groups (which also indicates successful randomisation). Furthermore, vitamin A and zinc levels in an acute illness may not reliably indicate micronutrient status.23 Reliable assessment would require a large study of population micronutrient status.23 Lastly, although we had 80% power to detect a clinically important difference in our primary outcome, our study had little power to detect small differences, especially in malnourished children. The secondary and interaction outcomes had less than 80% power.
The secondary outcomes (time to resolution of tachypnoea and fever) are often used as measures in ALRI studies in developing countries. These signs are less likely to be present at hospitalisation in our study population, as most children were medically evacuated from remote communities and received intramuscular antibiotic therapy (in accordance with protocols) before transfer, as well as paracetamol and oxygen. Hence, although most participants clearly had pneumonia on chest x-ray, relatively few had fever or tachypnoea on admission. Indeed, these differences in the acute hospitalisation phase limit the applicability of our findings to other populations, and from other populations to ours. The failure of the interventions in our population should not undermine efforts to improve nutrition and other contributors to ALRIs.
Our findings are relevant to the design of treatment protocols for Indigenous children in developed countries, as well as possibly to any future trials of zinc supplementation and vitamin A in affluent populations. Our findings do not support the use of vitamin A or zinc supplements in Indigenous children from remote areas who are hospitalised for ALRI. Even in populations with high rates of ALRI and poor living conditions, vitamin A and zinc supplements may not be useful; their effect may depend on the prevalence of deficiency of these micronutrients in the particular population.
2 Characteristics of infants and children with acute lower respiratory tract infection (ALRI) at entry into the trial, by treatment group (number of episodes)*
|
Zinc (n = 57) |
Vitamin A (n = 54) |
Zinc + vitamin A (n = 54) |
Placebo (n = 50) |
P |
||||||||||
Age (months) |
|
|
|
|
|
||||||||||
0–11 |
20 (35%) |
17 (32%) |
17 (32%) |
16 (32%) |
0.75 |
||||||||||
12–23 |
15 (26%) |
11 (20%) |
8 (15%) |
9 (18%) |
|
||||||||||
24–59 |
16 (28%) |
14 (26%) |
18 (33%) |
13 (26%) |
|
||||||||||
60 + |
6 (11%) |
12 (22%) |
11 (20%) |
12 (24%) |
|
||||||||||
Sex |
|
|
|
|
|
||||||||||
Male |
28 (49%) |
35 (65%) |
26 (48%) |
34 (68%) |
0.07 |
||||||||||
Female |
29 (51%) |
19 (35%) |
28 (52%) |
16 (32%) |
|
||||||||||
Household employment |
|
|
|
|
|
||||||||||
Mostly unemployed |
31 (61%) |
33 (72%) |
35 (78%) |
31 (67%) |
0.35 |
||||||||||
Even numbers |
14 (28%) |
6 (13%) |
8 (18%) |
11 (24%) |
|
||||||||||
Mostly employed |
6 (12%) |
7 (15%) |
2 (4%) |
4 (9%) |
|
||||||||||
Weight-for-age z score |
|
|
|
|
|
||||||||||
≤ − 2 (underweight) |
6 (11%) |
4 (7%) |
3 (6%) |
6 (12%) |
0.74 |
||||||||||
− 1 |
13 (23%) |
18 (33%) |
13 (25%) |
11 (22%) |
|
||||||||||
≥ 0 |
38 (67%) |
32 (59%) |
36 (69%) |
33 (66%) |
|
||||||||||
Weight-for-height z score |
|
|
|
|
|
||||||||||
≤ − 2 (wasted) |
1 (2%) |
3 (8%) |
2 (5%) |
7 (19%) |
0.09 |
||||||||||
− 1 |
12 (26%) |
9 (23%) |
10 (23%) |
4 (11%) |
|
||||||||||
≥ 0 |
33 (72%) |
28 (70%) |
32 (73%) |
26 (70%) |
|
||||||||||
Height-for-age z score |
|
|
|
|
|
||||||||||
≤ − 2 (stunted) |
5 (11%) |
4 (10%) |
2 (5%) |
1 (3%) |
0.51 |
||||||||||
− 1 |
4 (9%) |
6 (14%) |
8 (19%) |
8 (21%) |
|
||||||||||
≥ 0 |
38 (81%) |
32 (76%) |
33 (77%) |
29 (76%) |
|
||||||||||
Breastfeeding |
|
|
|
|
|
||||||||||
Still breastfeeding |
30 (63%) |
26 (58%) |
21 (54%) |
19 (44%) |
0.51 |
||||||||||
Stopped breastfeeding |
6 (13%) |
8 (18%) |
10 (26%) |
12 (28%) |
|
||||||||||
Never breastfed |
12 (25%) |
11 (24%) |
8 (21%) |
12 (28%) |
|
||||||||||
Anaemia (Hb < 110 g/L) |
30 (58%) |
23 (46%) |
26 (49%) |
21 (48%) |
0.65 |
||||||||||
Chest x-ray |
|
|
|
|
|
||||||||||
Normal |
2 (4%) |
2 (4%) |
4 (8%) |
0 |
0.14† |
||||||||||
Lobar pneumonia |
51 (90%) |
51 (96%) |
47 (90%) |
49 (98%) |
|
||||||||||
Bronchopneumonia |
3 (5%) |
0 |
1 (2%) |
0 |
|
||||||||||
Increased respiratory rate |
22 (39%) |
20 (38%) |
21 (40%) |
23 (47%) |
0.77 |
||||||||||
Fever (temperature ≥ 38.5°C) |
21 (37%) |
11 (20%) |
21 (39%) |
20 (40%) |
0.11 |
||||||||||
Pulse oximetry Spo2 < 95%‡ |
13 (23%) |
8 (15%) |
7 (13%) |
9 (18%) |
0.54 |
||||||||||
Penicillin treatment§ |
46 (81%) |
43 (80%) |
45 (83%) |
39 (78%) |
0.92 |
||||||||||
* Figures are number of ALRI episodes. Some data were missing. Children who were readmitted with ALRI more than 120 days after the first admission were eligible to re-enrol. † Fisher’s exact test. ‡ Spo2 < 95% (lowest limit of reference range) = hypoxaemia. § Penicillin treatment after enrolment. Hb = haemoglobin. |
|||||||||||||||
3 Outcomes in infants and children with acute lower respiratory tract infection (ALRI) after supplementation with zinc, vitamin A or both, compared with placebo
|
Vitamin A supplementation |
Zinc supplementation |
Zinc + vitamin A |
||||||||||||
|
Yes |
No |
P |
Yes |
No |
P |
Yes |
No |
P |
||||||
Child days* |
12 960 |
12 840 |
|
13 320 |
12 480 |
|
6 480 |
6 000 |
|
||||||
Median (range) |
|
|
|
|
|
|
|
|
|
||||||
Days with Spo2 < 95%†‡ |
0 (0–6) |
0 (0–3) |
0.47 |
0 (0–6) |
0 (0–3) |
0.98 |
0 (0–6) |
0 (0–5) |
0.51 |
||||||
Hours to resolution of feverठ|
11.0 (2–76) |
12.0 (2–69) |
0.90 |
12.0 (3–76) |
11.0 (2–57) |
0.70 |
10.0 (3–76) |
12.0 (2–69) |
0.71 |
||||||
Hours for respiratory rate to settle¶ |
17.0 (2–60) |
14.0 (3–120) |
0.84 |
22.0 (4–69) |
12.0 (2–120) |
0.36 |
22.0 (4–56) |
13.5 (2–120) |
0.63 |
||||||
Days in hospital |
5.0 (2–46) |
5.0 (1–25) |
0.10 |
5.0 (1–46) |
5.0 (1–25) |
0.75 |
5.0 (2–46) |
5.0 (1–25) |
0.27 |
||||||
ALRI readmissions within 120 days** |
11 (10%) |
11 (10%) |
|
16 (14%) |
6 (6%) |
|
8 (15%) |
14 (9%) |
|
||||||
Relative risk (RR) of readmission (95% CI)†† |
1.0 (0.5–2.1)‡‡ |
|
|
2.4 (1.003–6.1)‡‡ |
|
|
1.6 (0.7–3.7)§§ |
|
|
||||||
RR (95% CI) (interaction term included)¶¶ |
0.9 (0.2–4.4) |
|
|
2.2 (0.6–7.8) |
|
|
2.5 (0.7–9.2) |
|
|
||||||
* Days from first day of admission to end of 120-day follow-up. † Spo2 < 95% (lowest limit of reference range) = hypoxaemia. ‡ Data were missing for 2 children. § Temperature < 37.5°C. ¶ Respiratory rate below cut-off value (60 breaths per min at age < 2 months, 50 breaths per min at 2–11 months, 40 breaths per min at 12–59 months, and 30 breaths per min at > 5 years). ** From first day of admission. †† Relative risks (RRs) were calculated using generalised estimating equation model (binomial) and adjusted for sex. ‡‡ Reference groups for the vitamin A group and for the zinc group comprised children who received either placebo or the alternative micronutrient alone. In each case, RR was adjusted for supplementation with the other micronutrient. §§ Reference group for vitamin A + zinc comprised children who received placebo only (no zinc and no vitamin A); RR was not adjusted. ¶¶ RRs were also determined for supplementation as two independent variables (zinc yes/no, vitamin A yes/no) versus placebo (no zinc and no vitamin A) with an interaction term, zinc–vitamin A supplementation, included in the model. The interaction term was not significant (P = 0.89). |
|||||||||||||||
Received 3 May 2005, accepted 10 October 2005
- Anne B Chang1
- Paul J Torzillo2
- Peter M Stewart3
- Naomi C Boyce4
- Andrew V White5
- Gavin R Wheaton6
- David M Purdie7
- John Wakerman8
- Patricia C Valery9
- 1 Flinders University Northern Territory Clinical School and Royal Children's Hospital, Brisbane, QLD.
- 2 Royal Prince Alfred Hospital, Sydney, NSW.
- 3 Flinders University Northern Territory Clinical School, Alice Springs, NT.
- 4 Northern Territory Department of Health and Community Services, Alice Springs, NT.
- 5 Alice Springs Hospital, Alice Springs, NT.
- 6 Queensland Institute of Medical Research and School of Population Health, University of Queensland, Brisbane, QLD.
- 7 Flinders University and Charles Darwin University, Alice Springs, NT.
- 8 Queensland Institute of Medical Research, Population Studies and Human Genetics and Centre for International and Tropical Health and Nutrition, University of Queensland, Brisbane, QLD.
We thank the children and their families who participated in the study, Valerie Logan for technical support, and Alison Furber for helping to obtain consent.
The study was partly funded by the Sylvia and Charles Viertel Charitable Foundation (Anne Chang’s Clinical Investigatorship), a Flinders University Research Grant and Flinders University NT Clinical School. Anne Chang is funded by a National Health and Medical Research Council Practitioner Fellowship and the Royal Children’s Hospital Foundation. None of the funding bodies had any role in study design, data collection and analysis, or writing of the manuscript.
None identified.
- 1. d’Espaignet ET, Kennedy K, Paterson BA, Measey ML. From infancy to young adulthood: health status in the Northern Territory. Darwin: Territory Health Services, 1998.
- 2. Chang AB, Masel JP, Boyce NC, Torzillo PJ. Respiratory morbidity in central Australian Aboriginal children with alveolar lobar abnormalities. Med J Aust 2003; 178: 490-494. <MJA full text>
- 3. Yamey G. Zinc supplementation prevents diarrhoea and pneumonia. BMJ 1999; 319: 1521-1522.
- 4. Bhutta ZA, Black RE, Brown KH, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators’ Collaborative Group. J Pediatr 1999; 135: 689-697.
- 5. Villamor E, Mbise R, Spiegelman D, et al. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics 2002; 109: E6.
- 6. Brown N, Roberts C. Vitamin A for acute respiratory infection in developing countries: a meta-analysis. Acta Paediatr 2004; 93: 1437-1442.
- 7. Christian P, West KP. Interactions between zinc and vitamin A: an update. Am J Clin Nutr 1998; 68 (2 Suppl): S435-S441.
- 8. Smith JC, McDaniel EG, Fan FF, Halsted JA. Zinc: a trace element essential in vitamin A metabolism. Science 1973; 181: 954-955.
- 9. Rahman MM, Vermund SH, Wahed MA, et al. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ 2001; 323: 314-318.
- 10. Porea TJ, Belmont JW, Mahoney DH. Zinc-induced anemia and neutropenia in an adolescent. J Pediatr 2000; 136: 688-690.
- 11. Valery PC, Torzillo PJ, Boyce NC, et al. Zinc and vitamin A supplementation in Australian Indigenous children with acute diarrhoea: a randomised controlled trial. Med J Aust 2005; 182: 530-535. <MJA full text>
- 12. World Health Organization. Cough or difficult breathing. IMCI (Integrated Mangement of Childhood Illness) referral guide: management of the child with a serious infection or severe malnutrition. Geneva: WHO, 2000.
- 13. Stephensen CB, Franchi LM, Hernandez H, et al. Adverse effects of high-dose vitamin A supplements in children hospitalized with pneumonia. Pediatrics 1998; 101: E3.
- 14. Brooks WA, Yunus M, Santosham M, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet 2004; 363: 1683-1688.
- 15. Ruel MT, Rivera JA, Santizo MC, et al. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics 1997; 99: 808-813.
- 16. Black RE. Therapeutic and preventive effects of zinc on serious childhood infectious diseases in developing countries. Am J Clin Nutr 1998; 68: S476-S479.
- 17. Bogden JD. Influence of zinc on immunity in the elderly. J Nutr Health Aging 2004; 8: 48-54.
- 18. Sugarman B,Epps LR. Zinc and Chlamydia trachomatis. Proc Soc Exp Biol Med 1985; 179: 382-387.
- 19. Mustafa MG, Cross CE, Munn RJ, Hardie JA. Effects of divalent metal ions on alveolar macrophage membrane adenosine triphosphatase activity. J Lab Clin Med 1971; 77: 563-571.
- 20. Griffiths JK. The vitamin A paradox. J Pediatr 2000; 137: 604-607.
- 21. Si NV, Grytter C, Vy NN, et al. High dose vitamin A supplementation in the course of pneumonia in Vietnamese children. Acta Paediatr 1997; 86: 1052-1055.
- 22. Nacul LC, Kirkwood BR, Arthur P, et al. Randomised, double blind, placebo controlled clinical trial of efficacy of vitamin A treatment in non-measles childhood pneumonia. BMJ 1997; 315: 505-510.
- 23. Srinivas U, Braconier JH, Jeppsson B, et al. Trace element alterations in infectious diseases. Scand J Clin Lab Invest 1988; 48: 495-500.






Abstract
Objective: To evaluate the efficacy of supplementation with zinc and vitamin A in Indigenous children hospitalised with acute lower respiratory infection (ALRI).
Design: Randomised controlled, 2-by-2 factorial trial of supplementation with zinc and vitamin A.
Setting and participants: 187 Indigenous children aged < 11 years hospitalised with 215 ALRI episodes at Alice Springs Hospital (April 2001 to July 2002).
Interventions: Vitamin A was administered on Days 1 and 5 of admission at a dose of 50 000 IU (infants under 12 months), or 100 000 IU; and zinc sulfate was administered daily for 5 days at a daily dose of 20 mg (infants under 12 months) or 40 mg.
Main outcome measure: Time to clinical recovery from fever and tachypnoea, duration of hospitalisation, and readmission for ALRI within 120 days.
Results: There was no clinical benefit of supplementation with vitamin A, zinc or the two combined, with no significant difference between zinc and no-zinc, vitamin A and no-vitamin A or zinc + vitamin A and placebo groups in time to resolution of fever or tachypnoea, or duration of hospitalisation. Instead, we found increased morbidity; children given zinc had increased risk of readmission for ALRI within 120 days (relative risk, 2.4; 95% CI, 1.003–6.1).
Conclusion: This study does not support the use of vitamin A or zinc supplementation in the management of ALRI requiring hospitalisation in Indigenous children living in remote areas. Even in populations with high rates of ALRI and poor living conditions, vitamin A and zinc therapy may not be useful. The effect of supplementation may depend on the prevalence of deficiency of these micronutrients in the population.