Stroke is the second-largest cause of death in developed countries and the major cause of disability.1 Of the estimated 44 000 people who have strokes each year in Australia,2 a third will die within a year,2 and almost half of the survivors will be disabled.3 Organised stroke services reduce mortality and dependence compared with conventional stroke care,4 and stroke units, in which a multidisciplinary team provides focused management in a geographically localised area, are the gold standard.5 In Australia, about 90% of stroke patients are treated in hospital,6 but only 23% of hospitals have a formal stroke service.7
The Australian guidelines for stroke units include patients being placed in a geographically defined unit; the presence of a coordinated multidisciplinary team (stroke physician, nursing staff, occupational therapist, physiotherapist, speech pathologist, dietitian, social worker and, where possible, psychologist); access to ongoing professional education; regular team meetings for care and discharge planning; and use of agreed evidence-based management protocols.8 Smaller hospitals may lack these resources, but efforts are made to provide the necessary care. A mobile stroke service (MSS),4 involving a multidisciplinary team that cares for stroke patients dispersed throughout a hospital, can be an intermediary step for many centres.
In 1998, a prospective, multicentre evaluation of stroke services (Stroke Care Outcomes: Providing Effective Services [SCOPES]) commenced in Australia.9 SCOPES evaluated clinical practices, models and outcomes of care in an effort to provide evidence and delineate factors that make stroke units more effective. Fifteen quality indicators were identified. These included:
activities within 24 hours: brain imaging, swallowing assessment, allied health assessment, neurological observations;
documentation of premorbid function and discharge needs; and
management practices: enteric feeding if nil orally for more than 48 hours, measures to avoid aspiration, deep-vein thrombosis (DVT) prophylaxis, fever management and use of antiplatelet medication at discharge, as well as occupational therapy, speech therapy and physiotherapy within 48 hours.
Stroke units perform better than other models of care in adherence to quality indicators, and participants whose treatment adheres to all applicable indicators are more likely to be alive at discharge.9
In 2000, an MSS was started at a 260-bed medical centre in the eastern suburbs of Melbourne. The primary aim of our study was to use the quality assessment framework from SCOPES as part of an audit process. We compared quality of care following the introduction of the MSS with historical controls. The audit results were then benchmarked against the SCOPES stroke units9 as a method of providing a quality assurance program for future stroke management at our hospital.
Our retrospective, observational study was designed to measure adherence to quality indicators when our 260-bed hospital provided conventional care (September 1998 to October 1999) compared with adherence two years after implementing an organised MSS (July 2002 to June 2003). Secondary comparisons included utilisation of diagnostic investigations, complication rates, mortality, independence at discharge and length of stay. Comparisons were also made between our MSS and stroke units assessed in SCOPES.10
Patients were identified via the hospital database using ICD-9-CM (430–480) and ICD-10-AM (I60–I64, G45–G46) discharge coding for stroke. Sample-size estimates of 100 patients per group were based on the detection of a change in adherence from 52% to 72% (mean adherence rates observed for conventional ward care versus MSS care in SCOPES9) for a single quality indicator, giving 80% power with α = 0.05.
SCOPES subject eligibility criteria were used.9 All adults presenting with a stroke within 3 days of symptom onset were included. Transient ischaemic attacks (TIAs), subarachnoid haemorrhages and in-hospital strokes were excluded.
Strokes were classified into subtypes using the Oxfordshire Community Stroke Project Classification.11 The modified Rankin score12 was used as a measure of functional status before stroke and at discharge. Stroke severity was assessed using established predictors of outcome, including upper-limb strength, verbal deficits and urinary incontinence.13 Complications were classified according to SCOPES criteria: mild (easily tolerated symptoms that did not interfere with treatment or rehabilitation), moderate (causing significant discomfort, requiring increased monitoring) or severe (life-threatening events that prolonged hospital stay).10
Statistical analyses were performed using SPSS for Windows14 and Epi Info Statcalc.15 Categorical data were analysed using χ2 tests, and skewed continuous variables using the non-parametric Mann–Whitney U test. To reflect care that should have been given, rates of adherence were reported after taking into account whether the indicators were applicable in each case. Comparisons of outcomes were made on the basis of intention to treat. Appropriate adjustments for casemix variation were undertaken to compare the rates of adherence and outcomes between the control and MSS groups.13
Three hundred and seventeen consecutive patient records were screened until 100 patients per group were reached. Sixty-five patients were excluded from the MSS group, and 52 from the control group. The main reasons for exclusion were non-stroke (MSS, 16; control, 20) and transient ischaemic attack (MSS, 17; control, 10).
The groups were similar in demography, risk factors and stroke characteristics (Box 1), and in prognostic factors13 that predict potential discharge rates after stroke (MSS, 79%; control, 82%; OR, 0.98; 95% CI, 0.51–1.91).
Adherence to most quality indicators was better after instituting the MSS compared with the control period (Box 2). Compared with stroke units, adherence in our MSS was lower for early allied health assessments, incontinence management, fever management and appropriate use of antiplatelet therapy.
Severe and moderate complications were fewer in the MSS group, with more patients in the MSS group independent at discharge compared with the control group (Box 3). Discharge destination for most MSS group patients was home with support (home rehabilitation, community services).
Overall in-hospital mortality was similar in both groups, with stroke the most common cause of death (MSS, 18%; control, 11%; OR, 3.82; 95% CI, 0.67–24.08). Palliative care was instituted in similar numbers in both groups (MSS, 16%; control, 8%; OR, 2.19; 95% CI, 0.83–5.92). The median length of stay for survivors in the MSS group was reduced significantly.
Carotid ultrasonography, echocardiography and MRI scans were used more frequently in the MSS group to delineate stroke subtype and stroke mechanism.
Stroke care at this hospital was substantially improved, with subsequent reductions in severe complications and length of hospital stay, after formation of the MSS. By comparing our current indicator adherence results with those of stroke units, we have identified areas for future quality improvement initiatives, such as early allied health assessment and appropriate secondary stroke prophylaxis with antiplatelet therapy.
Accurate diagnosis of stroke type and mechanism is important, as these have implications for long-term outcome and risk of stroke recurrence.16 Carotid doppler ultrasound, computed tomography brain imaging and transthoracic echocardiography were available at the hospital in both periods. The hospital does not have on-site facilities for MRI or transoesophageal echocardiography. The increased use of these investigations reflects the increased vigilance by the stroke physician in determining cause to enhance preventive and treatment strategies.
Discharge disability and destination varied substantially between the two groups. Although home rehabilitation programs have been in place since 1998, intensive home rehabilitation programs only started in August 1999. The number of beds available doubled in 2000, and regular in-service education sessions have taken place. This might partly explain why more patients were discharged home with support services in the MSS group.
Limitations of this study include reporting bias, where activities that formed our quality indicators may have been carried out but not documented, or where researchers applied subjective judgements. Completion of care pathways in 47% of MSS group patients made assessment of adherence to indicators easier. Conversely, adherence may have been underestimated in the control group. The study was not powered to detect differences in outcome, yet the differences were statistically significant. In some instances, such as utilisation of MRI, small numbers affected statistical accuracy.
As we have emphasised, localised stroke units are the gold standard. However, not all hospitals have the required resources. We initiated the stroke service with one neurologist employed to provide neurological services 5 hours a week. There were no other increases in resources, despite plans for extra allied health staff, increased training for nursing staff and specialised equipment. The support and enthusiasm of the hospital staff made it possible.
We suggest that a localised stroke unit with the institution of a stroke liaison nurse and increased specialist physician sessions would lead to a better stroke service in this hospital. Regular in-service education in acute stroke management is needed, especially as the hospital has rotating medical registrars from other hospitals, as many peripheral centres do.
It is possible to create an effective mobile stroke service in a smaller hospital. With the publication of studies such as SCOPES, we can also institute audits that assess both quality of care and the more traditional markers of hospital care. Audits could be repeated annually, as an update for continued stroke service improvement and to enable benchmarking against the gold standards of stroke units. This work re-emphasises the need for stroke patients to be managed by a dedicated service.
1 Baseline characteristics
|
Mobile Stroke Service (n = 100) |
Control Group (n = 100) |
P |
||||||||||||
Demographics |
|
|
|
||||||||||||
Median (interquartile range) age (years) |
78.3 (71.6–86.1) |
79.9 (72.5–85.9) |
0.774 |
||||||||||||
Female |
54 |
52 |
0.770 |
||||||||||||
Lives alone |
18 |
30 |
0.047 |
||||||||||||
Independent before stroke* |
71 |
69 |
0.758 |
||||||||||||
Risk factors |
|
|
|
||||||||||||
Smoking |
43/93 (46%) |
51/99 (52%) |
0.464 |
||||||||||||
Atrial fibrillation |
34 |
36 |
0.766 |
||||||||||||
Hypertension |
70 |
69 |
0.878 |
||||||||||||
Diabetes mellitus |
22 |
18 |
0.480 |
||||||||||||
Previous stroke/TIA |
46 |
43 |
0.115 |
||||||||||||
Stroke subtype (Oxfordshire11) |
|
|
|
||||||||||||
Total anterior circulation infarct‡ |
17/81 (21%) |
15/89 (17%) |
0.491 |
||||||||||||
Partial anterior circulation infarct‡ |
34/81 (42%) |
32/89 (36%) |
0.421 |
||||||||||||
Posterior circulation infarct‡ |
11/81 (14%) |
10/89 (11%) |
0.665 |
||||||||||||
Lacunar infarct‡ |
19/81 (24%) |
31/89 (35%) |
0.104 |
||||||||||||
Haemorrhage |
19/100 (19%) |
11/100 (11%) |
0.113 |
||||||||||||
Stroke severity13 |
|
|
|
||||||||||||
Ability to lift arms |
55 |
63 |
0.250 |
||||||||||||
Walking |
21 |
18 |
0.592 |
||||||||||||
Verbal deficits† |
43 |
47 |
0.570 |
||||||||||||
Incontinence |
48 |
51 |
0.671 |
||||||||||||
* Independence (Modified Rankin Score12 0–2: No symptoms at all to slight disability). †Abnormal Glasgow Coma Score verbal component. ‡Calculated as fraction of ischaemic strokes. TIA = transient ischaemic attack. |
|||||||||||||||
2 Adherence to quality indicators
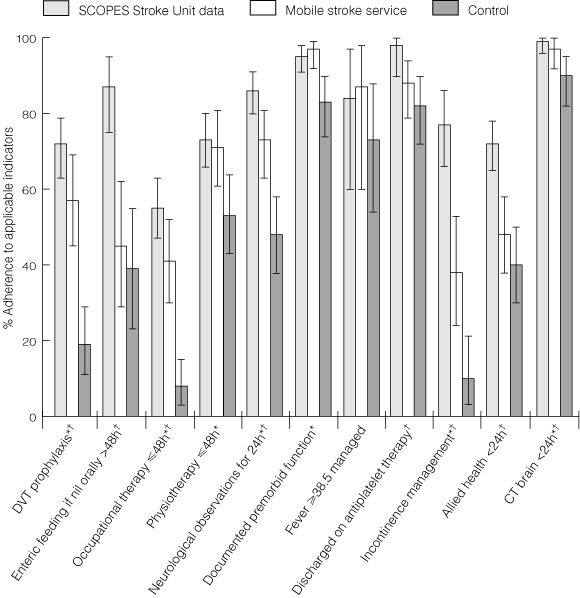
* P < 0.05 related to control group and mobile stroke service data comparison only. †P < 0.05 related to mobile stroke service and SCOPES stroke unit data comparison only. Error bars represent 95% confidence intervals.
3 Special investigations and acute care outcomes
|
Mobile Stroke Service (n = 100) |
Control Group (n = 100) |
Odds ratio (95% CI) |
P |
|||||||||||
Special investigations |
|
|
|
|
|||||||||||
Brain CT scan |
99 |
96 |
4.13 (0.45–37.57) |
0.174 |
|||||||||||
Brain MRI |
18 |
2 |
10.76 (2.44–97.39) |
< 0.001 |
|||||||||||
Carotid ultrasonography |
63 |
41 |
2.45 (1.33–4.51) |
0.002 |
|||||||||||
Transthoracic echocardiography |
48 |
27 |
2.50 (1.33–4.71) |
0.002 |
|||||||||||
Transoesophageal echocardiography |
15 |
1 |
17.47 (2.34–361.87) |
< 0.001 |
|||||||||||
Complications* |
|
|
|
|
|||||||||||
Any complication |
34 |
56 |
0.41 (0.23–0.72) |
0.002 |
|||||||||||
Mild |
10 |
17 |
0.54 (0.24–1.23) |
0.148 |
|||||||||||
Moderate |
23 |
36 |
0.53 (0.29–0.98) |
0.044 |
|||||||||||
Severe |
9 |
24 |
0.31 (0.14–0.70) |
0.004 |
|||||||||||
Functional status on discharge† |
|||||||||||||||
Independent |
32 |
9 |
4.76 (2.01–11.55) |
< 0.001 |
|||||||||||
Death |
21 |
18 |
1.13 (0.54–2.40) |
0.592 |
|||||||||||
Discharge destination |
|
|
|
|
|||||||||||
Home with support |
33 (42%) |
10 (12%) |
4.43 (1.93–10.39) |
< 0.001 |
|||||||||||
Rehabilitation facility |
27 (34%) |
34 (42%) |
0.72 (0.37–1.37) |
0.282 |
|||||||||||
Nursing home |
12 (15%) |
19 (23%) |
0.58 (0.25–1.35) |
0.171 |
|||||||||||
Length of stay‡ (days) |
|
|
|
|
|||||||||||
Discharged alive (median [interquartile range]) |
12.0 (7–17) (n = 79) |
18.5 (9–33) (n = 82) |
|
0.003 |
|||||||||||
All (median [interquartile range]) |
9.5 (5.5–15) |
17.5 (8–33) |
|
< 0.001 |
|||||||||||
* Patients with at least one complication. †Independence (Modified Rankin Score12 0–2: No symptoms at all to slight disability). ‡Length of stay calculated as median stay of survivors, and overall length of stay. CT = computed tomography. MRI = magnetic resonance imaging. |
|||||||||||||||
Received 15 July 2004, accepted 21 December 2004
- Anneke van der Walt1
- Amanda K Gilligan2
- Amy G Brodtmann3
- Dominique A Cadilhac4
- Dora C Pearce5
- Geoffrey A Donnan6
- 1 Department of Medicine, The Alfred Hospital, Prahran, VIC.
- 2 Eastern Neurosciences, Box Hill Hospital, Box Hill, VIC.
- 3 National Stroke Research Institute, Heidelberg Heights, VIC.
This study was supported by the National Stroke Research Institute. We would like to thank Jacqui Lye, who assisted with development of the stroke service, nursing education in stroke care, data collection and feedback on early analysis in this study. We would also like to thank Maria Di Pietro, Michelle Fox, Tamara Clements, Jason Faux and Karen Martin for their contribution as research officers in the original SCOPES team, and Sonia Morissey, Penny Bisset, and LiChun Quang for their contribution to database management, as well as John Ludbrook for assistance with sample size calculation.
None identified.
- 1. Murray C, Lopez A. Global mortality, disability, and the distribution of risk factors: global burden of disease study. Lancet 1997; 349: 1436-1442.
- 2. Thrift AG, Dewey HM, MacDonell RAL, et al. Stroke incidence on the east coast of Australia: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2000; 31: 2087-2092.
- 3. Bamford J, Sandercock P, Dennis M, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981–86. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral haemorrhage and sub-arachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990; 53: 16-22.
- 4. Stroke Unit Trialists’ collaboration. Organised inpatient (stroke unit) care for stroke (Cochrane Review). In: The Cochrane Library. Oxford: Update software. Issue 1, 2003.
- 5. Evans A, Perez I, Harraf F, et al. Can differences in management processes explain different outcomes between stroke unit and stroke team care? Lancet 2001; 358: 1586-1592.
- 6. Gilligan AK, Thrift AG, Dewey HM, et al. Hospital arrival following stroke onset: a population based study [abstract]. Cerebrovasc Dis 2001; 11 (Suppl 4): 11.
- 7. Ogden K, Cadilhac D, Pearce D, et al. Access to organised stroke care in Australia: results of a national survey of acute stroke treating hospitals [abstract]. Cerebrovasc Dis 2001; 11 (Suppl 4): abstract 93.
- 8. National clinical guidelines for acute stroke management. Melbourne: National Stroke Foundation, 2003. Available at: www.strokefoundation.com.au/ (accessed Jan 2005).
- 9. Cadilhac D, Ibrahim J, Pearce D, et al. Multicenter comparison of processes of care between stroke units and conventional care wards in Australia. Stroke 2004; 35: 1035-1040.
- 10. National Stroke Foundation. Stroke care outcomes: providing effective services (SCOPES) — an evaluation of Victorian stroke services. Melbourne: National Stroke Foundation, 2002.
- 11. Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991; 337: 1521-1526.
- 12. Bamford J, Sandercock P, Warlow C, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989; 20: 828.
- 13. Counsell C, Dennis M, McDowell M, Warlow C. Predicting outcome after acute and sub-acute stroke: development and validation of new prognostic models. Stroke 2002; 33: 1041-1047.
- 14. SPSS for Windows [computer program]. Version 11.5. Chicago, Ill: SPSS Inc, 2003.
- 15. Epi Info Statcalc [computer program]. Version 6. Atlanta: Centers for Disease Control, 1994.
- 16. Hankey G, Jamrozik K, Broadhurst R, et al. Long-term risk of recurrent stroke in the Perth Community Stroke Study. Stroke 1998; 29: 2491-2500.





Abstract
Objective: An Australian stroke services study (SCOPES) has developed a framework to compare different forms of acute stroke services, the gold standard being localised stroke units. We aimed to use this framework to assess changes in the quality of stroke care over time as a sequential audit process.
Design and setting: A retrospective medical record audit comparing 100 sequential stroke admissions (July 2002 to June 2003) two years after institution of a mobile stroke service (MSS) with 100 historical controls (September 1998 to October 1999) at a 260-bed hospital in Melbourne. The MSS results were also compared with stroke units in SCOPES.
Main outcome measures: Adherence to quality indicators and standard measures of outcome (complications, length of stay and discharge disability) after implementing the MSS.
Results: Significant improvements were seen in prophylaxis for deep-vein thrombosis, incontinence management, premorbid function documentation, frequent neurological observations and early occupational therapy. The MSS demonstrated fewer severe complications (9% versus 24%; P = 0.004), reduced median length of stay (discharged patients: 12.0 days versus 18.5 days; P = 0.003) and more patients were independent at discharge (32% versus 9%; P < 0.001). Comparison with SCOPES stroke units showed our MSS could improve in incontinence management and appropriate use of antiplatelet therapy.
Conclusion: Institution of the MSS was associated with improvements in the quality of stroke care. This study demonstrates application of an audit procedure for quality improvement in hospital stroke management and the potential to improve stroke services in smaller centres.