Total hip replacement (THR) surgery, as we know it, began in 1960.1 Total knee replacement (TKR), as currently performed, was first described in 1971.2 While these operations are very successful, deep-vein thrombosis (DVT) and pulmonary embolism (PE) have been significant complications. The reported prevalence of DVT in patients not receiving prophylaxis in clinical trials using mandatory venography has been 45%–57% after THR3-6 and 40%–84% after TKR.7-10 The reported prevalence of PE in other trials has been 0.7%–30% after THR11-15 (prevalence of fatal PE, 0.1%–0.4%), and 1.8%–7% after TKR (prevalence of fatal PE, 0.2%–0.7%).16,17
Lower-limb and respiratory symptoms and signs can be difficult to assess in the first few days after THR and TKR, making the clinical diagnosis of DVT and PE unsatisfactory.
Ultrasonography has improved greatly in recent years and is now capable of detecting even calf DVT satisfactorily. We believe it is suitable for pre-discharge screening of large numbers of patients after lower-limb joint replacement. Ventilation/perfusion nuclear scanning (V/Q scanning) is well established in the diagnosis of PE, with computed tomographic pulmonary angiography performed on selected patients in whom diagnostic doubt exists after a V/Q scan. In 1995, these investigations were readily available in our hospital.
The aim of our study was to determine the prevalence of venous thromboembolism (VTE) after THR, TKR or bilateral TKR in a large patient sample in a single institution over an extended period. The surgical procedures were accompanied by short-term chemical and physical prophylaxis. The diagnostic techniques used were ultrasonography, V/Q scanning and computed tomographic pulmonary angiography. To our knowledge, such a study had not been done before in Australia, with most reports in the literature describing multicentre studies involving relatively small numbers of patients at each institution.
Our management approach is summarised in the algorithm in Box 1. If no DVT was found within 7 days, the patient was discharged to home or a rehabilitation hospital with no chemoprophylaxis against VTE, although most surgeons advised patients to wear graduated compression elastic stockings for 6 weeks.
All results were recorded daily, entered into a database, and reviewed at 3-monthly intervals throughout the study.
Odds ratios for the differences in risk of DVT between patients receiving different forms of chemical and physical prophylaxis were calculated using standard χ2 methods.18 P values were 2-sided (α = 0.05). Although a small number of patients had knee replacements at different times in the course of the study, the proportion of “double counting” in the TKR group was small (3%) and was not considered significant.
The number of patients developing DVT within 7 days postoperatively was 263/3028 (8.9%) after THR, 627/2441 (25.6%) after TKR, and 196/530 (36.9%) after bilateral TKR (Box 2).
Non-fatal. Of 250 V/Q scans performed, 115 (1.9%) were positive for PE (Box 2).
Fatal. Three fatal pulmonary emboli occurred among the 5999 patients in the study (a prevalence of 0.05%). The three deaths were all after knee replacements.
One man, aged 75, with a history of PE after appendicectomy and herniorrhaphy as a young man and an uneventful TKR in 1991, had a TKR in 1998 during our study. Multiple pulmonary emboli were detected on Day 7 postoperatively, associated with massive DVT. He died on Day 23.
A second man, aged 84, with a history of mild chronic obstructive airway disease, developed a pulmonary infection after a TKR during our study. An initial V/Q scan was negative, but a second scan late in the illness was positive. He also suffered a myocardial infarction and died on Day 36.
A third man, aged 76, a chronic smoker with bronchiectasis, collapsed 5 days after bilateral TKR and could not be resuscitated. PE was thought to be the probable cause of death.
Risk of any DVT after different types of surgery. The risk of DVT was greater after TKR than THR (odds ratio [OR], 3.64; 95% CI, 3.12–4.26; P < 0.001), and much greater after bilateral TKR than THR (OR, 6.17; 95% CI, 4.97–7.66; P < 0.001). The risk was also greater after bilateral TKR than TKR (OR, 1.69; 95% CI, 1.39–2.07; P < 0.001). The cumulative risk of DVT for two TKRs in the one patient, weeks or months apart, was greater than for bilateral TKR.
Risk of thigh DVT after different types of surgery. Thigh DVT was much more likely after THR than either TKR (OR, 4.19; 95% CI, 2.59–6.77; P < 0.001) or bilateral TKR (OR, 6.89; 95% CI, 2.88–16.47; P < 0.001).
Risk of any DVT after different types of prophylaxis. The risk of DVT was much less in patients who had intermittent calf compression in the first 7 days postoperatively than patients who did not (Box 3).
The majority of patients (88%) were given LMWH prophylaxis for the first 7 days postoperatively, with only 12% given warfarin. The risk of DVT was greater in patients taking warfarin than those taking LMWH (Box 4). However, this result should be interpreted with caution, as the choice of anticoagulant was made by the surgeon rather than randomly assigned.
There was no excess of bleeding, infection or wound complication associated with prophylactic anticoagulation therapy.
The principal strengths of our study were that it involved a large cohort of consecutive patients in a single institution over a period of nearly 7 years. We also had the advantage of retaining, throughout the study, the same radiologist (I A B) to supervise ultrasound scans and the same chief ultrasonographer.
A limitation of our study was that it did not have controls to enable comparison between different forms of management. Thus, for example, no conclusions can be drawn from our study about whether ultrasonography is as effective as venography for detecting DVT, or whether LMWH is better than warfarin for prophylaxis.
Also, patients without DVT on ultrasound at Day 7 were not restudied for DVT. Although we think it is uncommon, it is possible some of these patients may have developed new thrombi after leaving hospital on Day 7, in which case we may have underestimated the prevalence of DVT.
The 6th American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy reviewed numerous studies of VTE involving various prophylactic measures.15 In 30 trials of LMWH prophylaxis (involving a total of 6216 patients), total DVT prevalence after THR was 16.1%, and in 13 trials (involving a total of 1740 patients) total DVT prevalence after TKR was 30.6%. The corresponding prevalences in our study — 8.9% and 26.9%, respectively — were lower than the ACCP prevalences.
Chemical and physical prophylactic measures may have an additive effect on VTE reduction, and this may account for the lower prevalence of VTE in our patients, all of whom received both forms of prophylaxis.
Our patients were studied by ultrasonography, while the patients in the ACCP review were studied by venography. It has been said that ultrasonography is less sensitive than venography, and, in a 1995 meta-analysis, Wells et al concluded, for that reason, that ultrasonography had limitations as a screening test after orthopaedic surgery.16 However, the studies on which that conclusion was based were done between 1982 and 1993. In 1994, Oishi et al found that, for detection of DVT after THR and TKR, ultrasonography had 100% sensitivity, 98% specificity and 98% accuracy in men and 88% sensitivity, 98% specificity and 97% accuracy in women.19 Since then, ultrasound technology has improved, and calf DVT can be well seen and measured to the nearest centimetre.
The ACCP review did not recommend routine ultrasonography at the time of hospital discharge or during outpatient follow-up in asymptomatic THR or TKR patients. It quoted the study of Leclerc et al,17 who concluded that “pre-discharge compression ultrasound cannot be justified”. However, that study used a “limited ultrasound method that [had] been shown to be sensitive to proximal vein thrombosis in symptomatic patients”, suggesting that the method was insensitive for detecting distal DVT.
Leclerc et al found that only 3/1936 (0.2%) of THR and TKR patients who received in-hospital LMWH prophylaxis had asymptomatic DVT on pre-discharge ultrasonography. Of our 4874 THR and TKR patients taking prophylactic LMWH, 719 developed DVT (14.8%). In view of the huge discrepancy between the data of Leclerc and colleagues and ours, we believe that their ultrasonography technique may have been inadequate for detecting calf DVT. This would have resulted in a much lower prevalence of total DVT than in our study, where 82%–97% of deep-vein thrombi were in the calf. Leclerc et al also excluded patients with a previous history of VTE, whereas we had no exclusions.
Presumably, if PE occurs without any DVT being found in the leg, the PE has either come from the pelvis, or a thrombus from the leg has completely embolised to the lung. However, if a patient has calf DVT and PE, it is possible there may have been an additional thrombus in the pelvis or thigh that embolised completely, leaving the patient with PE and calf DVT. We believe it is also possible, especially when there is extensive calf DVT, that some of the calf thrombus may become detached and cause PE with residual calf DVT.
It may be that with intensive prophylaxis over the years, the pattern of leg DVT is changing, with a higher proportion of calf thrombi than previously. This was suggested by Ciccone et al in 1998.20 In a number of studies in the early 1990s, the proportion of calf deep-vein thrombi relative to total deep-vein thrombi in patients not receiving prophylaxis was 30%–50% after THR and 70%–75% after TKR.15 In our study, with prophylaxis, the total prevalence of DVT was much lower (18.1%), and the proportion of calf thrombi was higher (83% after THR and 95% after TKR). It may be that, with current technology, we can better detect calf DVT, or it may mean that prophylaxis not only reduces the prevalence of total thrombi but also results in a preponderance of calf thrombi. We know that calf thrombi can extend, and if they become thigh thrombi would be more likely to cause PE.
In assessing whether a deep-vein thrombus in the leg will embolise, the total length of thrombus may be important. The mean total length of thrombus in our patients was 10 cm, but the mean total thrombus length in those with symptomatic in-hospital PE was 19 cm, suggesting that total thrombus length may be important in embolisation.
Planes et al found that the risk of late DVT after THR is high until Day 35 postoperatively if prophylaxis is not continued after leaving hospital.21 A study by White et al found that the median time of diagnosis of symptomatic DVT is Day 17 after THR and Day 7 after TKR.22 Comp et al showed that a 4-week course of enoxaparin prophylaxis after THR gave therapeutic benefit, reducing the incidence of VTE without compromising safety (however, a similar benefit was not seen in TKR patients).23
Given the uncertain behaviour of post-operative deep-vein thrombi, we believe that pre-discharge ultrasonography is helpful. It assists in planning post-discharge VTE management, an important point in our situation, where many patients face long trips home by car or plane, often to small centres with limited medical facilities. There is some evidence that ultrasonography will detect nearly all deep-vein thrombi by Day 7: in 200 consecutive THR and TKR patients showing no DVT on ultrasound at Day 7 managed with the same protocol as ours in a similar hospital in Sydney the prevalence of DVT on ultrasound at Day 90 was only 1% (Michael McGrath, Vascular Physician, St Vincent’s Hospital, Sydney, personal communication). This would suggest that post-discharge “symptomatic” DVT is almost always visible on ultrasonography by Day 7. Whatever the case, the concept of “symptomatic” and “asymptomatic” DVT is flawed, as nearly all patients have pain and swelling in the leg (especially the calf) after THR or TKR, making accurate, early clinical diagnosis of DVT impossible.
Further studies comparing the VTE status of all patients at 90 days postoperatively with their status at 7 days would help in understanding the pathogenesis of VTE after lower-limb joint replacement.
Finally, while many deep-vein thrombi are relatively harmless, some are associated with PE, which, in rare instances, can be fatal. The dilemma is in knowing which thrombi are the dangerous ones.
1 Management algorithm for patients having THR, TKR or bilateral TKR at the Mater Misericordiae Hospital, North Sydney, NSW, Apr 1995–Dec 2001
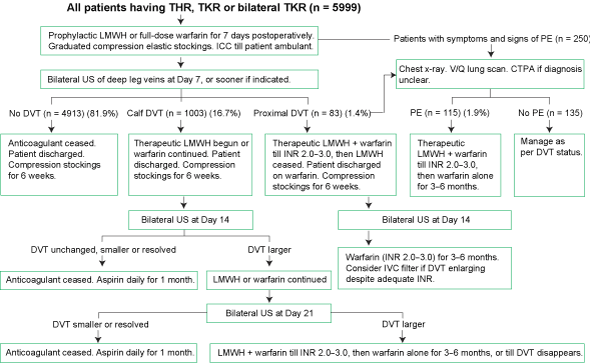
CTPA = computed tomographic pulmonary angiography. DVT = deep vein thrombosis. ICC = intermittent calf compression. INR = international normalised ratio. IVC = inferior vena cava. LMWH = low-molecular-weight heparin. PE = pulmonary embolism. THR = total hip replacement. TKR = total knee replacement. US = ultrasonography. V/Q lung scan = ventilation/perfusion lung scan.
2 Pre-discharge prevalence of DVT and pulmonary embolism after hip or knee replacement surgery (total n = 5999)
|
After THR (n = 3028) |
After TKR (n = 2441) |
After bilateral TKR (n = 530) |
Total |
|||||||||||
Total number of patients with DVT |
263/3028 (8.9%) |
627/2441 (25.6%) |
196/530 (36.9%) |
1086/5999 (18.1%) |
|||||||||||
Patients with distal DVT |
217/263 (82.5%) |
596/627 (95.1%) |
190/196 (96.9%) |
1003/1086 (92.4%) |
|||||||||||
Patients with proximal DVT |
46/263 (17.5%) |
31/627 (4.9%) |
6/196 (3.1%) |
83/1086 (7.6%) |
|||||||||||
Patients with contralateral DVT (ie, in non-operated leg) |
55/263 (20.9%) |
56/627 (8.9%) |
n/a |
|
|||||||||||
Proportion of deep-vein thrombi larger at Day 14 despite 7 days’ treatment* |
7.0% |
10.0% |
7.0% |
|
|||||||||||
Proportion of deep-vein thrombi same size, smaller or absent at Day 14 after 7 days’ treatment* |
93.0% |
90.0% |
93.0% |
|
|||||||||||
Symptomatic in-hospital pulmonary embolism (n = 115) |
37/3028 (1.2%) |
68/2441 (2.8%) |
10/530 (1.9%) |
115/5999 (1.9%) |
|||||||||||
Patients with three or more deep-vein thrombi |
13/263 (4.9%) |
75/627 (12.0%) |
35/196 (17.9%) |
|
|||||||||||
DVT = deep vein thrombosis. THR = total hip replacement. TKR = total knee replacement. * Based on 528 (49%) patients with thrombus. |
|||||||||||||||
3 Proportion of patients with DVT after 7 days, according to whether they received intermittent calf compression (ICC) or no calf compression*
|
With ICC |
Without ICC |
Odds ratio (95% CI) |
P |
|||||||||||
After THR |
6.9% |
17.5% |
0.34 (0.26–0.45) |
< 0.0001 |
|||||||||||
After TKR |
23.2% |
42.1% |
0.41 (0.32–0.52) |
< 0.0001 |
|||||||||||
After bilateral TKR |
34.5% |
58.5% |
0.37 (0.21–0.66) |
0.0011 |
|||||||||||
DVT = deep vein thrombosis. THR = total hip replacement. TKR = total knee replacement. * ICC was not introduced until June 1996 (15 months after the beginning of the study). |
|||||||||||||||
4 Proportion of patients with DVT after 7 days, according to whether they received warfarin or low-molecular-weight heparin (LMWH)*
|
Warfarin |
LMWH |
Odds ratio (95% CI) |
P |
|||||||||||
After THR |
12.5% |
8.6% |
1.49 (0.94–2.38) |
< 0.0115 |
|||||||||||
After TKR |
35.5% |
23.6% |
1.79 (1.43–2.24) |
< 0.0001 |
|||||||||||
After bilateral TKR |
47.1% |
33.9% |
1.73 (1.14–2.61) |
0.0118 |
|||||||||||
DVT = deep vein thrombosis. THR = total hip replacement. TKR = total knee replacement. * The choice of anticoagulant was made by the surgeon rather than randomly assigned. |
|||||||||||||||
Received 13 May 2004, accepted 17 November 2004
- Richard F O’Reilly1
- Ian A Burgess2
- Bernard Zicat3
- Mater Misericordiae Hospital, North Sydney, NSW.
We would like to thank Professor Tony Keech and Dr Greg King for assistance with statistics, Dr Bob Cooper for performing the ventilation/perfusion nuclear scans, and all three for assistance with the manuscript. We also thank Ms Melissa Ambrose for data entry.
Richard O’Reilly has received speaker fees and travel assistance to attend meetings from AstraZeneca.
- 1. Charnley J. Arthroplasty of the hip: a new operation. Lancet 1961; 1: 1129-1132.
- 2. Gunston FH. Total knee arthroplasty. J Bone Joint Surg Br 1971; 53B: 272-277.
- 3. Turpie AGG, Levine MN, Hirsh J, et al. A randomised controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med 1986; 315: 925-929.
- 4. Hull RD, Raskob GE, Gent M, et al. Effectiveness of intermittent pneumatic leg compression for preventing deep vein thrombosis after total hip replacement. JAMA 1990; 263: 2313-2317.
- 5. Lassen MR, Borris LC, Christiansen HM, et al. Prevention of thromboembolism in 190 hip arthroplasties. Comparison of LMW heparin and placebo. Acta Orthop Scand 1991; 62: 33-38.
- 6. Hoek JA, Nurmohamed MT, Hamelynck KJ, et al. Prevention of deep vein thrombosis following total hip replacement by low molecular weight heparinoid. Thromb Haemost 1992; 67: 28-32.
- 7. Cohen SH, Erhlich GE, Kauffman MS, et al. Thrombophlebitis following knee surgery. J Bone Joint Surg Am 1973; 55: 106-112.
- 8. Stullberg BN, Insall JN, Williams GW, et al. Deep-vein thrombosis following total knee replacement: an analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am 1984; 66: 194-201.
- 9. Lynch AF, Bourne RB, Rorabeck CH, et al. Deep-vein thrombosis and continuous passive motion after total knee arthroplasty. J Bone Joint Surg Am 1988; 70: 11-14.
- 10. Stringer MD, Steadman CA, Hedges AR, et al. Deep vein thrombosis after elective knee surgery. J Bone Joint Surg Br 1989; 71: 492-497.
- 11. Eriksson BI, Kalebo P, Anthmyr BA, et al. Prevention of deep-vein thrombosis and pulmonary embolism after total hip replacement: comparison of low-molecular-weight heparin and unfractionated heparin. J Bone Joint Surg Am 1991; 73: 484-493.
- 12. Mohr DN, Silverstein MD, Ilstrup DM, et al. VTE associated with hip and knee arthroplasty: current prophylactic practices and outcomes. Mayo Clin Proc 1992; 67: 861-870.
- 13. Warwick D, Williams MH, Bannister GC, et al. Death and thromboembolic disease after total hip replacement: a series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br 1995; 77: 6-10.
- 14. Murray DW, Britton AR, Bulstrode CJK. Thromboprophylaxis and death after total hip replacement. J Bone Joint Surg Br 1996; 78: 863-870.
- 15. Geerts W, Heit J, Clagett G, et al. Prevention of venous thromboembolism. Chest 2001; 119(1Suppl): 132S-175S.
- 16. Wells PS, Lensing AW, Davidson BL, et al. Accuracy of ultrasound for the diagnosis of deep venous thrombosis in asymptomatic patients after orthopaedic surgery: a meta-analysis. Ann Intern Med 1995; 122: 47-53.
- 17. Leclerc JR, Gent M, Hirsh J, et al. The incidence of symptomatic venous thromboembolism during and after prophylaxis with enoxaparin: a multi-institutional cohort study in patients who underwent hip or knee arthroplasty. Arch Intern Med 1998; 158: 873-878.
- 18. SPSS for Windows version 11.5. Chicago, Ill: SPSS Inc, 2002.
- 19. Oishi CS, Grady-Benson JC, Otis SM, et al. The clinical course of distal deep venous thrombosis after total hip and total knee arthroplasty, as determined with duplex ultrasonography. J Bone Joint Surg Am 1994; 76: 1658-1663.
- 20. Ciccone WJ, Fox PS, Neumyer M, et al. Ultrasound surveillance for asymptomatic deep venous thrombosis after total joint replacement. J Bone Joint Surg Am 1998; 80: 1167-1174.
- 21. Planes A, Vochelle N, Darmon J-Y, et al. Risk of deep-venous thrombosis after hospital discharge in patients having undergone total hip replacement: double-blind randomised comparison of enoxaparin versus placebo. Lancet 1996; 348: 224-228.
- 22. White RH, Romano PS, Zhou H, et al. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 1998; 158: 1525-1531.
- 23. Comp PC, Spiro TE, Friedman RJ, et al. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. J Bone Joint Surg Am 2001; 83-A: 336-345.





Abstract
Objective: To determine the prevalence of venous thromboembolism (VTE) after total hip replacement (THR), total knee replacement (TKR) or bilateral TKR in a large sample of patients in a major hospital orthopaedic unit.
Design, setting and patients: The Mater Misericordiae Hospital, North Sydney, NSW, a 195-bed private hospital. All patients who had THR, TKR or bilateral TKR at the hospital between 1 April 1995 and 31 December 2001 had physical prophylaxis (graduated compression elastic stockings or intermittent pneumatic compression, or both) and chemical prophylaxis (anticoagulant) against VTE. All underwent ultrasonography of both legs before discharge, with a small, symptomatic group also undergoing a ventilation/perfusion lung scan (V/Q scan) and computed tomographic pulmonary angiography.
Main outcome measures: Prevalence of deep-vein thrombosis (DVT) and symptomatic pulmonary embolism (PE) before discharge.
Results: Among a total of 5999 patients, the pre-discharge prevalence of DVT after THR, TKR or bilateral TKR was 8.9%, 25.6% and 36.9%, respectively. The prevalence of symptomatic non-fatal in-hospital PE was 1.9%, while the prevalence of fatal in-hospital PE was 0.05%.
Conclusions: Despite short-term chemical and physical thromboprophylaxis, the prevalence of DVT after lower-limb joint replacement, measured by pre-discharge ultrasonography, was high. The rate of symptomatic non-fatal in-hospital PE was moderate, but fatal in-hospital PE was rare.