Indigenous Australians have high rates of coronary artery heart disease1 and die from this condition at more than twice the rate of other Australians.2 Ensuring equal access to optimal care is a key objective of the free public hospital system in Australia, but good information about the extent to which this is being achieved in Indigenous Australians is scarce, in particular as regards optimal care for acute coronary syndromes — percutaneous coronary intervention (PCI) and bypass surgery.
Cunningham found that Indigenous patients admitted to public hospitals for circulatory disease were 47% less likely than other patients to have a diagnostic or therapeutic procedure.3 The only other published information, a recent report by the Australian Institute of Health and Welfare (AIHW), found that for the period 1998–2001, population-based PCI rates were 40% lower among Indigenous men than non-Indi-genous men, but were 20% higher among Indigenous women than non-Indigenous women.4 For bypass surgery, the corresponding figures were 7% lower for men and 50% higher for women.
One limitation of the AIHW report was that, in calculating the rates, Indigenous status identification in the numerator (number of PCIs and bypass operations) may be different from Indigenous status identification in the denominator (population data from the Australian Bureau of Statistics). This problem is not unique to comparisons of Indigenous and non-Indi-genous Australians; it hinders interpretation of race-specific rates around the world.5,6
Here, we report a cohort study based on linkage of computerised discharge abstracts. The two cohorts for comparison were Indigenous and non-Indigenous patients treated in the public sector for acute myocardial infarction (AMI). We followed up each patient for 1 year to determine whether they had an invasive coronary procedure. Because the comparisons are based on data from a single source, they are not affected by differential classification of Indigenous status in the numerator and denominator.
We used administrative hospital data7 of patients with AMI treated in the public sector in Queensland between 1998 and 2002. Our cohort consisted of patients admitted through an emergency department (to ensure the identified admission was for the acute episode) whose principal diagnosis was subsequently coded as AMI: 410.x1 (ICD-9-CM)8 or I21.x (ICD-10-AM).9 As in other similar studies, only the first admission for AMI for each patient in the 5-year period was retained for analysis.10
We defined private-sector patients as those whose index admission was to a private hospital (or they were transferred to a private hospital during their index admission) and those who subsequently had a PCI or bypass surgery in a private hospital (Box 1).
Each patient was followed up for 1 year using probabilistic matching of computerised discharge abstracts based on data items such as date of birth, sex, country of birth and address of usual residence.11 We also linked the administrative hospital data to death registration data. Consequently, we were able to identify those patients with AMI who died either in hospital or outside, and we were able to censor these observations when calculating procedure rates.
We applied two exclusion criteria: age younger than 30 years or older than 89 years; and a discharge status of alive and length of stay fewer than 4 days. These criteria have been shown in other studies to reduce the number of false-positive diagnoses of AMI in administrative hospital data.12
Adjusted rates for PCI and bypass surgery performed during the index admission (where there was no time component) were obtained using generalised linear models with a binomial distribution and a log link.13
Rate ratios comparing time to subsequent PCI or bypass surgery (where there was a time component) were obtained using proportional-hazards models. Besides allowing adjustment for covariates, these models accounted for censoring due to death.14
The study cohort consisted of 14 683 patients with AMI, of whom 558 (3.8%) identified as Indigenous. Patients in the Indigenous cohort were very different from those in the non-Indigenous cohort (Box 2). On average, they were 14 years younger and were more likely to be of low socioeconomic status, come from a remote area, and be admitted to a small hospital, staffed by a non-specialist and without facilities for coronary procedures.
After adjusting for age differences between the two cohorts, Indigenous patients were more likely than non-Indigenous patients to have comorbidities reported, notably chronic renal failure and diabetes (Box 3, Box 4).
After adjusting for age, Indigenous patients with AMI who survived long enough to be admitted to hospital were 79% (95% CI, 48%–116%) more likely to die over the ensuing 12 months (Box 5). The age-adjusted 1-year survival was 86.7% for non-Indigenous patients and 77.5% for Indigenous patients.
The adjusted rates for PCI during the index admission were significantly lower by 39% (rate ratio [RR], 0.61) among Indigenous compared with non-Indigenous patients (Box 6). Adjusted rates for subsequent PCIs were 28% lower (RR, 0.72). Adjusted rates for bypass surgery were similar among Indigenous and non-Indigenous patients with AMI. For any coronary procedure (ie, PCI or bypass surgery), the adjusted rates were significantly lower by 22% among Indigenous patients.
Box 7 shows the effect of the covariates available in our data set on the probability of having a coronary procedure. The results are presented separately for Indigenous and non-Indigenous patients and, because of the relatively small number of Indigenous patients, we have only presented the results for all coronary procedures combined. That is, separate results for PCI and bypass surgery for Indigenous patients with AMI were imprecise (ie, had extremely wide confidence intervals). For the same reason, we have not presented individual comorbidities, but instead combined them. The results show the expected pattern for age (probability of having a procedure decreases with age); sex (men more likely to have a pro-cedure than women); socioeconomic status (affluent people more likely to have a pro-cedure, even in the public sector); and comorbidites (people with at least one comorbidity less likely to have a procedure).
Our study is the first Australian analysis, based on administrative data, examining racial differences in coronary procedure rates. More than 25 such studies have been published in the United States.10 We found relatively low rates of PCI for Indigenous patients with AMI, even after adjusting for age, sex, remoteness, socioeconomic status, hospital characteristics and comorbidities. Adjusted rates for bypass surgery were more equitable, but adjusted rates for any cor-onary procedure (ie, PCI or bypass surgery) were 22% lower among Indigenous patients.
Moreover, the case-fatality rates for Indi-genous people show that they are a group at high risk of further coronary events. There are particularly strong indications for revascularisation procedures in such patients.17 Consequently, the lower rates of revascularisation procedures in Indigenous patients may indicate a greater inequity than suggested by our analyses, which do not take into account the potential benefit from the procedure.
There are at least four possible reasons why Indigenous patients with AMI have relatively low rates of coronary procedures in the public hospital sector, despite their clinical need. First, relative contraindications such as comorbidities, smoking and obesity might be more common. Second, Indigenous people might prefer not to have a procedure. Third, Indigenous people might have less access to coronary pro-cedures because they are more likely to live in remote areas or attend small hospitals. Fourth, there might be discrimination against them, albeit subtle, in the public hospital system.10
Like similar studies based on administrative data in the United States, our linked data set contained some of these factors, but not others. Specifically, we had data on socioeconomic status, remoteness, hospital characteristics, and comorbid conditions. Like similar studies in the United States, we adjusted for these covariates in the analysis to obtain the most valid estimates of differences in procedure rates, given the available data. However, we did not have data on potentially important variables such as severity of AMI, smoking status, and patient preferences for intervention. Consequently, we could not (and did not attempt to) untangle the possible causes of the disparity in procedure rates. We stress that the variables we did consider are not confounders in the traditional sense of being nuisance variables that should be ignored after being accounted for in a statistical model. Instead, they are of intrinsic interest because they lie on the causal pathway,18 providing clues about ways to address the problem.
Along these lines, the results for comorbidities are particularly helpful. Diabetes, chronic renal failure and pneumonia were at least twice as common among Indigenous patients with AMI as among non-Indigenous patients with AMI; and chronic bronchitis and emphysema and heart failure were at least 60% more common (Box 4). If a patient had at least one comorbidity, then their probability of having a coronary pro-cedure was reduced by 40% (Box 7). High levels of comorbidities have also been found among African Americans in the United States, and commentators there have suggested that improved access to primary care would allow for better management of comorbidites and, consequently, increased rates of revascularisation.19
There is some evidence that primary care can reduce the prevalence and severity of comorbidities among Indigenous Australians. A study in the Northern Territory showed significant improvements in rates of progression to renal failure by implementing evidence-based secondary prevention in a primary health care setting,20 and there have been similar encouraging results from an evaluation of a primary care program for diabetes in North Queensland.21
Two other features of our data are of particular interest. First, Indigenous patients hospitalised for AMI were 14 years younger than their non-Indigenous counterparts. This is consistent with data from other sources, which show that Indigenous people aged in their 50s have rates of chronic disease similar to non-Indigenous Australians aged in their 60s or 70s.2
Second, Indigenous patients who survive long enough to be admitted to hospital and receive medical treatment have case-fatality rates that are nearly twice as high as their non-Indigenous counterparts. Possible reasons include more severe disease or more life-threatening comorbidities. Our data set is not suited to the task, but further investigation is warranted to untangle the possible causes, because each is potentially amenable to modification. In particular, more accessible and culturally appropriate primary health care might enable Indigenous people with coronary artery disease to be identified earlier in the course of their illness and have any comorbidities treated. For example, mortality is 30 times higher in patients on dialysis than for other patients with AMI, even after adjusting for age, sex and the presence of diabetes.22 Also, Indigenous patients are known to be under-represented at cardiac rehabilitation programs,23 which can reduce cardiac deaths by up to 31%.24 In hospitals, previous work in Queensland has shown that low-cost quality improvement interventions can reduce mortality rates for AMI and might be especially effective in small hospitals where many Indi-genous patients are treated.25
Advances in technology, and in particular the use of stents for PCI, have improved success rates and increased the range of people who can benefit from coronary revascularisation.26 Therefore, increasing the capacity of the public sector to perform revascularisation might allow more Indi-genous patients with AMI and comorbidities to have these procedures, thereby delaying death, relieving symptoms and improving quality of life. However, there is no guarantee that Indigenous people would specifically benefit from this approach, especially if their prevalence of comorbidities remains high. For example, there is some evidence from Canada that physicians there generally select lower-risk patients for procedures after AMI.27 Also, in spite of a more than twofold increase in coronary revascularisation procedures in Finland between 1988 and 1996, inequalities remained, especially for the disadvantaged groups with a relatively high prevalence of comorbdities.28
Therefore, the extra capacity might be taken up treating more non-Indigenous patients with fewer comorbidities. What is needed is high-quality, integrated primary health care that complements the tertiary sector. The available data from the AIHW show that there is underfunding of primary care services for Indigenous Australians,29 and recent work done by Access Economics suggests that the underfunding is of the order of $300 million per year.30 The high rates of comorbidities found among the Indigenous patients in this study, and the effect of this on procedure rates, suggest that adequate primary health care is a pre-requisite for effective specialist care.
1 Definition of public and private sector patients

* Includes patients who were transferred to a private hospital during their index admission
2 Baseline characteristics of cohort. Data are percentage of patients (number) unless otherwise indicated
|
Indigenous (n = 558) |
Non-Indigenous (n = 14 125) |
|||||||||||||
Mean age (SD) |
54 years (13.2) |
68 years (13.1) |
|||||||||||||
Sex |
|
|
|||||||||||||
Men |
56.6% (316) |
64.3% (9090) |
|||||||||||||
Women |
43.4% (242) |
35.7% (5035) |
|||||||||||||
Socioeconomic status* |
|
|
|||||||||||||
High |
9.9% (55) |
22.7% (3209) |
|||||||||||||
Intermediate |
45.5% (254) |
50.3% (7101) |
|||||||||||||
Low |
44.6% (249) |
27.0% (3815) |
|||||||||||||
Remoteness of residence† |
|
|
|||||||||||||
Not remote |
76.2% (425) |
98.4% (13 900) |
|||||||||||||
Remote |
23.8% (133) |
1.6% (225) |
|||||||||||||
Hospital of index admission |
|||||||||||||||
Admissions for AMI per year |
|||||||||||||||
> 200 |
7.7% (43) |
26.5% (3749) |
|||||||||||||
101–200 |
15.8% (88) |
30.5% (4301) |
|||||||||||||
51–100 |
32.3% (180) |
28.0% (3955) |
|||||||||||||
< 51 |
44.3% (247) |
15.0% (2120) |
|||||||||||||
Facilities for invasive coronary procedures |
|||||||||||||||
Angiography and PCI ± bypass |
16.7% (93) |
21.7% (3063) |
|||||||||||||
Angiography only |
12.0% (67) |
8.6% (1221) |
|||||||||||||
No angiography |
71.3% (398) |
69.7% (9841) |
|||||||||||||
Distance to hospital with facilities for invasive coronary procedures |
|||||||||||||||
< 30 km |
25.1% (140) |
51.9% (7337) |
|||||||||||||
30–150 km |
13.8% (77) |
24.6% (3468) |
|||||||||||||
> 150 km |
61.1% (341) |
23.5% (3320) |
|||||||||||||
Specialty of admitting doctor |
|
||||||||||||||
Cardiologist |
32.3% (180) |
32.5% (4596) |
|||||||||||||
General physician |
32.6% (182) |
55.6% (7857) |
Non-specialist |
35.1% (196) |
11.8% (1672) |
||||||||||
* Socioeconomic status (SES) was based on “statistical local area” of usual residence. There are 466 such areas in Queensland (median population, 5500). A high or low SES area was defined as being in the top or bottom quartiles, respectively, based on educational level, skilled employment, and income in the 2001 census.15 †Based on the remoteness classification of the Australian Bureau of Statistics.16
3 Age standardised* prevalence of comorbidities† by Indigenous status
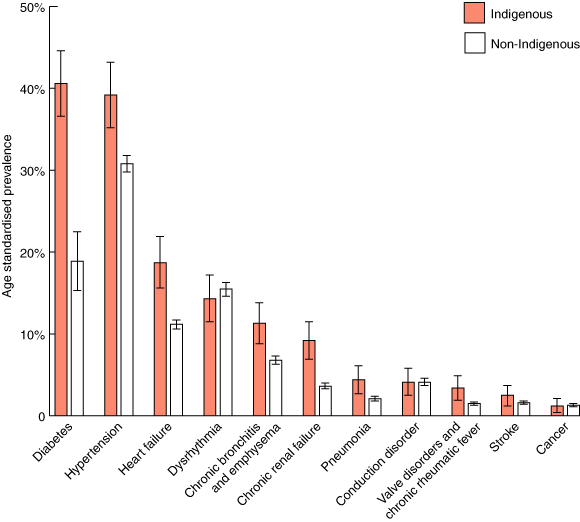
*To improve statistical precision, the much larger non-Indigenous cohort (mean age, 68 years) was age standardised to the age distribution of the Indigenous cohort (mean age, 54 years). †We used comorbidities from a study based on administrative hospital data in Ontario,12 and then checked it against Queensland data to confirm that no other comorbidities were commonly reported for Queensland patients with AMI. Dementia and liver disease were not considered, as only three Indigenous patients had either of these conditions.
4 Risk ratios comparing age-standardised prevalence of comorbidities for Indigenous v non-Indigenous patients with AMI
Comorbidities |
Age-adjusted risk ratio* (95% CI) |
||||||||||||||
Chronic renal failure |
2.52 (1.90–3.35) |
||||||||||||||
Diabetes |
2.49 (2.17–2.86) |
||||||||||||||
Pneumonia |
2.15 (1.42–3.25) |
||||||||||||||
Valve disorders and chronic rheumatic heart disease |
2.13 (1.33–3.41) |
||||||||||||||
Chronic bronchitis and emphysema |
1.67 (1.29–2.14) |
||||||||||||||
Heart failure |
1.64 (1.35–2.00) |
||||||||||||||
Stroke |
1.46 (0.85–2.50) |
||||||||||||||
Hypertension |
1.26 (1.10–1.44) |
||||||||||||||
Conduction disorders |
1.01 (0.67–1.52) |
||||||||||||||
Cancer |
0.99 (0.47–2.12) |
||||||||||||||
Dysrhythmia |
0.92 (0.74–1.13) |
||||||||||||||
* Age-adjusted using a generalised linear model with a binomial distribution and a log link The reference group was non-Indigenous patients.
AMI = acute myocardial infarction.
6 Adjusted* rate ratios for coronary procedures for Indigenous v non-Indigenous patients with acute myocardial infarction treated in the public sector
|
Number of procedures |
|
|
||||||||||||
|
Indigenous patients |
Non-Indigenous patients |
Adjusted* rate ratio† (95% CI) |
P |
|||||||||||
PCI during index admission |
14 |
655 |
0.61 (0.38–0.98) |
0.04 |
|||||||||||
Subsequent PCI |
60 |
1261 |
0.72 (0.54–0.96) |
0.03 |
|||||||||||
Bypass during index admission |
9 |
162 |
0.93 (0.70–1.25) |
0.64 |
|||||||||||
Subsequent bypass |
59 |
1289 |
1.01 (0.54–1.65) |
0.73 |
|||||||||||
All revascularisations |
142 |
3367 |
0.78 (0.64–0.94) |
0.01 |
|||||||||||
* Adjusted for age, sex, socioeconomic status, remoteness, hospital characteristics, and comorbidities (see Box 2 and Box 3). †Rate ratios < 1.0 indicate lower adjusted rates for Indigenous patients. PCI = percutaneous coronary intervention.
7 Rate ratios for probability of any coronary procedure within 12 months for selected covariates
|
Rate ratio (95% CI) |
||||||||||||||
Indigenous patients |
Non-Indigenous patients |
||||||||||||||
Age (years) |
|
|
|||||||||||||
30–49 |
1.00 |
1.00 |
|||||||||||||
50–59 |
0.67 (0.44–1.03) |
1.06 (0.94–1.19) |
|||||||||||||
60–-69 |
0.50 (0.29–0.84) |
0.80 (0.71–0.90) |
|||||||||||||
70–79 |
0.33 (0.15–0.72) |
0.43 (0.38–0.48) |
|||||||||||||
80–89 |
0.28 (0.07–1.13) |
0.06 (0.05–0.08) |
|||||||||||||
Sex |
|
|
|||||||||||||
Men |
1.00 |
1.00 |
|||||||||||||
Women |
0.81 (0.56–1.17) |
0.47 (0.43–0.52) |
|||||||||||||
Socioeconomic status |
|||||||||||||||
High |
1.00 |
1.00 |
|||||||||||||
Intermediate |
0.96 (0.11–2.91) |
0.92 (0.84–1.02) |
|||||||||||||
Low |
0.93 (0.19–2.56) |
0.84 (0.76–0.94) |
|||||||||||||
Remoteness |
|
||||||||||||||
Not remote |
1.00 |
1.00 |
|||||||||||||
Remote |
0.70 (0.45–1.11) |
1.33 (1.02–1.74) |
|||||||||||||
At least one comorbidity |
|
||||||||||||||
No |
1.00 |
1.00 |
|||||||||||||
Yes |
0.59 (0.42–0.87) |
0.60 (0.56–0.65) |
|||||||||||||
Received 7 February 2005, accepted 7 April 2005
- 1. Australian Bureau of Statistics. National Health Survey: Aboriginal and Torres Strait Islander results, Australia. Canberra: ABS, 2002. (Catalogue No. 4715.0.)
- 2. Australian Bureau of Statistics, Australian Institute of Health and Welfare. The health and welfare of Australia’s Aboriginal and Torres Strait Islander population. Canberra: ABS, 2003. (Catalogue No. 4704.0.)
- 3. Cunningham J. Diagnostic and therapeutic procedures among Australian hospital patients identified as Indigenous. Med J Aust 2002; 176: 58-62. http://www.mja.com.au/public/issues/176_02_210102/cun10341_fm.html
- 4. Australian Institute of Health and Welfare. Coronary revascularisation in Australia, 2000. Canberra: AIHW, 2003. (AIHW Catalogue No. AUS-35.)
- 5. Paradies Y, Cunningham J. Placing Aboriginal and Torres Strait Islander mortality in an international context. Aust N Z J Public Health 2002; 26: 11-16.
- 6. Kaufman J. How inconsistencies in racial classification demystify the race construct in public health statistics. Epidemiology 1999; 10: 101-103.
- 7. Manual for the Queensland Hospital Admitted Patients Data Collection. Brisbane: Queensland Health, 2004.
- 8. National Coding Centre. Australian version of the international classification of diseases, 9th revision, clinical modification (ICD-9-CM). Sydney: NCC (University of Sydney), 1996.
- 9. National Centre for Classification in Health. International statistical classification of diseases and related health problems, 10th revision, Australian modification. Sydney: NCCH (University of Sydney), 2002.
- 10. Kressin N, Petersen L. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med 2001; 135: 352-366.
- 11. Howe GR. Use of computerised record linkage in cohort studies. Epidemiol Rev 1998; 20: 112-121.
- 12. Tu J, Austin P, Naylor C, et al. Acute myocardial infarction outcomes in Ontario. In: Naylor C, Slaughter P, editors. Cardiovascular and services in Ontario. Toronto: Institute for Clinical Evaluative Sciences, 1999: 83-100.
- 13. Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986; 123: 174-184.
- 14. Cox DR. Regression models and life tables. J R Stat Soc B 1972; 34: 187-220.
- 15. Australian Bureau of Statistics. Census of population and housing: socio-economic indexes for areas (SEIFA). Canberra: ABS, 2004. (Catalogue No. 2039.0.)
- 16. Australian Bureau of Statistics. Australian standard geographic classification (ASGC). Canberra: ABS, 2004. (Catalogue No. 1216.0.)
- 17. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators (FRISC II Investigators). Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet 1999; 354: 708-715.
- 18. Rothman K, Greenland S. Modern epidemiology, Philadelphia: Lippincott-Raven, 1998.
- 19. Schneider EC, Leape LL, Weissman JS, et al. Racial differences in cardiac revascularisation rates: does “overuse” explain higher rates among white patients. Ann Intern Med 2001; 135: 328-337.
- 20. Hoy W, Baker P, Kelly A, Wang Z. Reducing premature death and renal failure in Australian Aborigines: a community-based cardiovascular and renal protective program. Med J Aust 2000; 172: 497-502. <MJA full text>
- 21. McDermott R, Schmidt B, Sinha A, Mills P. Improving diabetes care in the primary healthcare setting: a randomised cluster trial in remote Indigenous communities. Med J Aust 2001; 174: 497-502.
- 22. Foley RN, Parfrey PS, Sarnak, MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998 (Suppl 3); 32: S112-S119.
- 23. National Heart Foundation of Australia, Australian Cardiac Rehabilitation Association. Recommended framework for cardiac rehabilitation. Sydney: NHFA, ACRA, 2004.
- 24. Jolliffe JA, Rees K, Taylor RS, et al. Exercise-based rehabilitation for coronary heart disease (Cochrane review). Cochrane Database Syst Rev 2001; (1): CD001800.
- 25. Scott I, Coory M, Harper C. The effects of quality improvement interventions on inhospital mortality after acute myocardial infarction. Med J Aust 2001; 175: 465-470.
- 26. Gunn J, Grech ED, Crossman D, Cumberland D. New developments in percutaneous coronary intervention. BMJ 2003; 327: 150-153.
- 27. Alter DA, Naylor CD, Austin PC, Tu JV. Long term MI outcomes at hospitals with or without on-site revascularisation. JAMA 2001; 285: 2101-2108.
- 28. Hetemaa T, Keskimaki I, Manderbacka K, et al. How did the recent increase in the supply of coronary operations in Finland affect socioeconomic and gender equity in their use? J Epidemiol Community Health 2003; 57: 178-185.
- 29. Australian Institute of Health and Welfare. Expenditures on health services for Aboriginal and Torres Strait Islander People, 1998-99. Canberra: AIHW, 2001. (AIHW Catalogue No. IHW 7.)
- 30. Access Economics. Indigenous health workforce needs. A report for the Australian Medical Association. Sydney: Access Economics, 2004.






Abstract
Objective: To compare rates of percutaneous coronary interventions (PCI) and bypass surgery after acute myocardial infarction (AMI) in Indigenous and non-Indigenous patients.
Design: Cohort study of public-sector patients who were followed up for 1 year using administrative hospital data.
Participants and setting: We followed up 14 683 public-sector patients admitted to Queensland hospitals for AMI between 1998 and 2002. Of these, 558 (3.8%) identified as Indigenous.
Outcome measures: Rates of PCI and bypass surgery, adjusted for differences between the Indigenous and non-Indigenous cohorts according to age, sex, socioeconomic status, remote residence, hospital characteristics, and comorbidities.
Results: The adjusted rate for PCI during the index admission was significantly lower by 39% (rate ratio [RR], 0.61; 95% CI, 0.38–0.98) among Indigenous versus non-Indigenous patients with AMI; the adjusted rate for subsequent PCI was significantly lower by 28% (RR, 0.72; 95% CI, 0.54–0.96). Adjusted rates for bypass surgery were similar in the two cohorts. For any coronary procedure (ie, PCI or bypass surgery), the adjusted rate was significantly lower by 22% (RR, 0.78; 95% CI, 0.64–0.94) among Indigenous patients with AMI. Diabetes, chronic renal failure, pneumonia, and chronic rheumatic fever were at least twice as common among Indigenous patients with AMI as in the rest of the cohort, and chronic bronchitis and emphysema and heart failure were at least 60% more common. If a patient had at least one comorbidity, then their probability of having a coronary procedure was reduced by 40%.
Conclusions: There are likely to be several reasons for the lower rates of coronary procedures among Indigenous patients, but their high rates of comorbidities and the association of comorbidities with lower procedure rates was an important finding. As investment in primary care can reduce the prevalence and severity of comorbidities, we suggest that adequate primary health care is a prerequisite for effective specialist care.