The misuse of benzodiazepines among injecting drug users (IDUs) has been documented in many countries,1-6 and has been associated with serious harms. These include benzodiazepine dependence, increased risk of heroin overdose, increased risky injection behaviour,1,2,7-9 and injection-related health problems, including gangrene and, sometimes, limb amputation.10
Because of concerns about the rising incidence of injection-related harm associated with temazepam gel-filled capsules, the Australian Pharmaceutical Advisory Council recommended the capsules be restricted under the Pharmaceutical Benefits Scheme (PBS). Before 1 May 2002, temazepam 10 mg tablets and 10 mg capsules (Euhypnos, Nocturne, Normison, and Temaze) were subsidised on the PBS or could be obtained on private prescription. Temazepam 20 mg capsules could only be obtained on private prescription. From 1 May 2002, temazepam 10 mg capsules required an Authority prescription (ie, prior approval from the Health Insurance Commission) to allow subsidy on the PBS. Temazepam 10 mg tablets remained on the PBS and no authority was required. The temazepam 10 mg tablets, 10 mg capsules and 20 mg capsules continued to be available on private prescription (ie, they could still be prescribed by any doctor and purchased without PBS subsidy). The restriction was not designed to reduce benzodiazepine use per se, but to reduce the injection of temazepam capsules.
We aimed to assess the effects of the restriction of temazepam capsules by examining:
Rates of benzodiazepine prescribing and prescribing patterns of general practitioners before and after the policy change;
The injection of temazepam capsules by a sentinel group of regular IDUs, and their sources of these drugs, before and after the policy change; and
Self-reported injection-related harms after the policy change.
The study was conducted in New South Wales, the Northern Territory, Queensland, Tasmania and Victoria, as these states reported the highest levels of benzodiazepine injection among sentinel groups of IDUs.11,12
Data on PBS-subsidised medicines and estimates of non-subsidised medicines were examined for changes in prescribing patterns by benzodiazepine type. The estimated prescription data were calculated from continuous data on all prescriptions dispensed from a validated sample of community-based pharmacies. Inpatient hospital prescribing was not included.13 The PBS data are based on the date of supply or dispensing of prescriptions.
We examined data from the General Practice Research Network (GPRN) records on prescribing patterns of a random sample of general practitioners who use Medical Director software.14 Data from 348 GPs in NSW, QLD and VIC were examined. Numbers in the NT and TAS were too few for analysis.
Intervention ARIMA (Autoregressive Integrated Moving Average) time-series models were used to estimate the effect of the restriction on weighted numbers of GPRN prescriptions dispensed between 1999 and 2003. The effect of the restriction was modelled as a step or a change in slope. Transfer functions were applied to the step function to model its shape and long-term properties.15 Results of the time-series analysis set the context for surveys of IDUs’ benzodiazepine use. Confidence intervals (95%) were calculated for proportions. Categorical variables were analysed using χ2. Analysis was conducted using SPSS for Windows.16
There was a decrease of 393 370 PBS prescriptions for temazepam 10 mg capsules and an increase (368 951 prescriptions) for PBS temazepam 10 mg tablets from January–April to May–August 2002 (Box 1). There were smaller increases in PBS prescriptions of other tablet preparations during this time (data available on request).
Private (non-PBS) prescriptions of temazepam 10 mg capsules increased in May 2002 and stabilised in subsequent months (8350 in April, 14 006 in May, 10 291 in June) (Box 1). The 33% (10 334) increase in private temazepam 10 mg capsule prescriptions in May–August 2002 compared with January–April 2002 was substantially less than the decrease in PBS prescriptions (77%; 393 370). Private prescriptions for temazepam 20 mg capsules decreased by 1221 between January–April and May–August 2002.
GP prescribing patterns changed immediately following May 2002 (with a shift from capsule to tablet prescribing) (Box 2), but the overall rate of benzodiazepine prescribing did not. The overall rate ranged from 62 prescriptions per 1000 patient visits in May 2002 to 70 prescriptions per 1000 patient visits in January 2003.
There was a permanent 73% reduction in the number of capsule prescriptions dispensed. The decline occurred within 6 weeks of the restriction, indicating stability in prescriptions dispensed beyond that time.
There was some evidence in both the population trends and the prescribing patterns that the effect of the restriction began 1–2 weeks before its inception, probably due to pre-warning of GPs. Details of time-series analyses are available on request.
Frequency of benzodiazepine use (Box 3) was higher in the December sample, reflecting the requirement of use in the past month for entry into this sample. However, frequency of benzodiazepine use was similar when only past-month benzodiazepine users were compared (Box 3).
Most IDUs in the June and December samples reported oral use of benzodiazepine tablets. The proportions reporting only using temazepam capsules orally remained stable after the 1 May restriction (June, 10%; December, 14%) (Box 3).
In the June 2002 survey, fewer IDUs reported injecting benzodiazepines (capsules or tablets) in the month after the restriction (28%) than in the 4 months preceding the restriction (49%) (Box 3). This apparent decrease should be interpreted with caution, as it may reflect the shorter time period (one month) used to measure the behaviour. A third of the December sample reported injecting benzodiazepines in the preceding month.
Similar proportions in the June (8%) and December (9%) sample reported injecting benzodiazepine tablets in the preceding month. In both samples, the tablets most commonly reported to have been injected were Hypnodorm, Valium and Xanax.
In June, 54% of the national sample reported using temazepam capsules between January and April (before the restriction), and most of these (37% overall) reported injecting the capsules (Box 3). The proportion who used and injected temazepam capsules (46% and 32%, respectively) in the month before the December survey (6 months after the restriction) was not significantly different from those reporting use and injection (54% and 37%, respectively) in January–April, before the restriction (Box 3). Rates of injecting temazepam capsules in the month before interview were not significantly different in the two periods following the restriction (June, 22%; December, 32%).
Injection of temazepam capsules was more common than oral use in both samples, with the largest proportions of those using them reporting injection as the sole route of administration in the month before interview (June, 16%; December, 20%).
Half of both samples reported obtaining benzodiazepines from doctors by presenting with genuine symptoms (Box 4). Of those who sought a benzodiazepine prescription in the past month, 86% reported that they were successful every time. Most of the December sample reported obtaining benzodiazepines from one (73%) or two (15%) doctors.
About half the respondents in June (47%) and December (46%) reported that it was “easy” to obtain tablet benzodiazepines from doctors. A third reported it was “very easy” (June, 31%; December, 36%).
More participants in the June survey (71%) reported that it had become more difficult to obtain temazepam capsules from the doctor than in the December survey (34%). Even so, 24% and 31% of the June and December samples, respectively, reported access through practitioners as “easy” or “very easy”.
Half of the IDUs reported buying benzodiazepines on the street before and after the policy change, with a third reporting the purchase of capsules (Box 4). In December, 49% of respondents reported that capsules were “easy” or “very easy” to obtain on the street, although 60% reported that access had become more difficult in the previous 6 months.
Among the December sample, most benzodiazepine injectors reported injecting mainly into their arms (71%). Smaller groups reported injecting mainly in their hands (9%) or groin (7%).
Two-thirds of IDUs who injected benzodiazepines reported injection-related problems. The most commonly reported problems were prominent scarring or bruising, difficulty finding veins to inject into, and perceived benzodiazepine dependence (Box 5).
Benzodiazepine injectors were more likely to report difficulty finding veins to inject into than IDUs who did not inject benzodiazepines (56% compared with 40%; χ2 = 5.275, df = 1, P < 0.05).
Participants who reported injecting benzodiazepines more than once a week reported a higher number of injection-related problems than those injecting weekly or less (median 2 versus 1; U = 557.0; P < 0.05).
A large reduction occurred in the prescribing of temazepam 10 mg capsules, with a concomitant increase in temazepam 10 mg tablets, after the 1 May 2002 restriction in PBS subsidy. Nevertheless, IDUs still reported accessing temazepam capsules after the restriction through prescription and illicit sources, with substantial proportions reporting injection of these capsules in the month preceding interview. Most IDUs who injected benzodiazepines reported injection-related problems, suggesting that the harms associated with injecting benzodiazepines are of ongoing concern.
We assessed the effect of the policy on a sentinel group of IDUs. These IDUs may not be representative of all IDUs who use benzodiazepines. About half the IDUs reported they were largely obtaining their benzodiazepines legitimately. Small proportions presented with “fake symptoms” to obtain benzodiazepines from a doctor. Specific guidelines may be required to assist doctors with assessing need for benzodiazepines. Although there was a decrease in the proportion of IDUs reporting easy access to temazepam capsules from doctors, substantial proportions reported access remained easy. This suggests there were doctors who would provide temazepam 10 mg capsules with an authority, or temazepam capsules on private prescription. Specific strategies aimed at supporting doctors and assisting them to refine their prescribing practices may be required, including improving skills in patient assessment, provision of alternatives to benzodiazepines, and reductions in overall benzodiazepine prescribing.
A commonly reported problem among the December sample was difficulty finding veins to inject into, indicative of vascular damage. This damage is probably not solely due to benzodiazepine injection, but temazepam capsules can be particularly harmful.3 Benzodiazepine dependence was also commonly reported, and this needs to be adequately assessed and treated.
As few respondents reported only injecting tablets, it was difficult to examine differences in problems between “tablet only” and “capsule only” injectors. As temazepam capsules have been restricted further, it is important to monitor the injection of tablets and the associated harms. Our study did not show a substantial increase in the injection of tablets after publicly subsidised temazepam capsules were restricted. However, doctors may need to pay particular attention to the prescribing of rapid-acting formulations, including Hypnodorm (flunitrazepam) and Xanax (alprazolam), as these may replace temazepam among IDUs. These tablets, in addition to the widely available Valium (diazepam), were the most commonly reported tablets injected in this survey.
Although it is difficult to estimate the costs associated with the injection of benzodiazepines, this issue is a significant public health concern, as the more severe conditions associated with this practice, such as pulmonary microemboli and gangrene, are costly to treat. Additionally, benzodiazepine-using IDUs have been found to use healthcare services more than IDUs who do not use benzodiazepines, with benzodiazepine injectors reporting significantly more GP visits than oral users.17,18
The restriction in temazepam capsules has limited the availability of the easily injectable temazepam capsules in the community, but additional measures may need to be taken to further reduce harm among IDUs who continue to access and inject capsules that have been acquired illicitly (from diversion) and on doctors’ prescriptions. Evidence reported here suggests further restrictions are required to limit temazepam capsule injecting and its associated harms. Strategies such as the treatment of benzodiazepine dependence, the promotion of safer injecting practices, and more proactive education for users regarding vein care and the associated harms of injecting need to be considered in order to reduce misuse of benzodiazepines and limit the associated harms.
1 Number of private and subsidised prescriptions of temazepam capsules and tablets in Australia, May 2001 to Dec 2002*
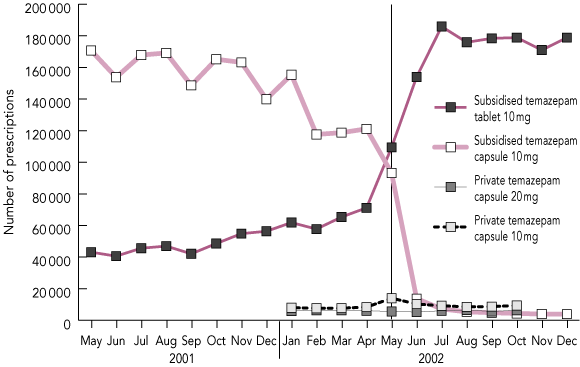
* Data for private temazepam prescriptions were only available for January to October 2002. Source: Drug Utilisation Subcommittee, Australian Department of Health and Ageing. The vertical line indicates the start of the capsule restrictions.
2 Rate of overall, capsule and tablet benzodiazepine prescribing by general practitioners, per 1000 patient visits per week in Australia, January 1999 to January 2003
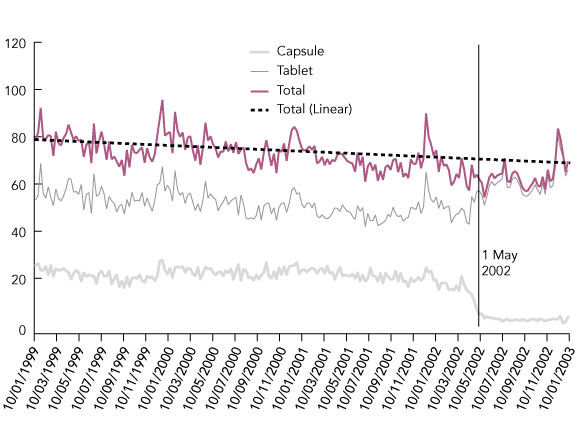
Source: General Practice Research Network database. The vertical line indicates the start of the capsule restrictions.
3 Benzodiazepine use among injecting drug users in the national June and December samples
|
June 2002 |
December 2002 |
|||||||||||||
|
Use between January and April (n = 350) |
Use in past month (n = 285) |
Use in past month (n = 255) |
||||||||||||
|
% |
95% CI |
% |
95% CI |
% |
95% CI |
|||||||||
Benzodiazepines |
|
|
|
|
|
|
|||||||||
Any oral use |
96 |
94–98 |
95 |
93–98 |
98 |
96–100 |
|||||||||
Oral only use |
51 |
46–56 |
72 |
66–77 |
67 |
62–73 |
|||||||||
Any injecting |
49 |
43–54 |
28 |
23–33 |
33 |
27–38 |
|||||||||
Injecting only |
4 |
2–6 |
5 |
3–8 |
2 |
1–3 |
|||||||||
Oral and injecting |
44 |
39–49 |
23 |
18–28 |
31 |
25–36 |
|||||||||
Daily users |
24 |
19–28 |
29 |
24–34 |
29 |
23–35 |
|||||||||
Benzodiazepine form |
|
|
|
|
|
|
|||||||||
Oral use of tablets* |
96 |
94–98 |
93 |
90–96 |
98 |
96–100 |
|||||||||
Injected tablets* |
11 |
1–15 |
8 |
5–12 |
9 |
6–13 |
|||||||||
Any temazepam capsule use |
54 |
49–59 |
32 |
27–37 |
46 |
40–52 |
|||||||||
Oral use temazepam capsules |
33 |
28–38 |
15 |
11–20 |
26 |
21–32 |
|||||||||
Injected temazepam capsules |
37 |
33–43 |
22 |
17–27 |
32 |
26–37 |
|||||||||
Temazepam oral only |
16 |
12–20 |
10 |
7–14 |
14 |
10–18 |
|||||||||
Temazepam injection only |
21 |
16–25 |
16 |
12–21 |
20 |
15–25 |
|||||||||
Temazepam oral and injection |
17 |
13–21 |
5 |
3–8 |
12 |
8–16 |
|||||||||
Frequency of benzodiazepine use |
|
|
|
||||||||||||
Median days used any benzodiazepine in the past 6 months |
30 (1–180) |
60 (1–180) |
72 (1–180) |
||||||||||||
* “Tablets” includes any benzodiazepine tablet formulation (including temazepam tablets). |
|||||||||||||||
4 Source of benzodiazepines reported by injecting drug users in June and December 2002
|
June 2002 |
December 2002 |
|||||||||||||
|
Use between January and April |
Use in past month |
Use in past month |
||||||||||||
|
% |
95% CI |
% |
95% CI |
% |
95% CI |
|||||||||
Main source of benzodiazepines (any form) |
(n = 350) |
(n = 285) |
(n = 255) |
||||||||||||
Doctors (genuine symptoms) |
53 |
47–58 |
52 |
47–58 |
55 |
49–61 |
|||||||||
Doctors (fake symptoms) |
9 |
6–12 |
6 |
3–9 |
3 |
1–5 |
|||||||||
Friends and family |
27 |
22–31 |
29 |
24–34 |
22 |
17–27 |
|||||||||
Street (illicit) |
11 |
7–14 |
12 |
8–16 |
18 |
13–22 |
|||||||||
Ease of obtaining temazepam capsules from a doctor* |
(n = 128) |
(n = 59) |
(n = 80) |
||||||||||||
Very easy |
32 |
23–39 |
10 |
2–18 |
11 |
4–18 |
|||||||||
Easy |
29 |
21–37 |
14 |
6–24 |
20 |
11–29 |
|||||||||
Difficult |
24 |
17–32 |
19 |
9–29 |
13 |
5–20 |
|||||||||
Very difficult |
15 |
9–22 |
59 |
47–72 |
34 |
23–44 |
|||||||||
Benzodiazepines obtained illicitly (“on the street”) |
(n = 350) |
(n = 285) |
(n = 255) |
||||||||||||
Any benzodiazepine |
50 |
45–55 |
52 |
46–58 |
55 |
48–61 |
|||||||||
Temazepam capsules |
30 |
25–35 |
21 |
16–25 |
32 |
26–37 |
|||||||||
10 mg capsules |
17 |
13–21 |
8 |
5–11 |
14 |
10–18 |
|||||||||
20 mg capsules |
21 |
17–25 |
14 |
10–18 |
26 |
20–31 |
|||||||||
Tablets† |
41 |
35–46 |
38 |
32–44 |
47 |
41–53 |
|||||||||
* Of those who requested temazepam capsules from a doctor (genuine or fake symptoms). † “Tablets” includes any benzodiazepine tablet formulation (including temazepam tablets). |
|||||||||||||||
5 Injection-related problems in the month before interview by benzodiazepine injectors* in the December sample
|
Injected any benzodiazepine (n = 83) |
||||||||||||||
|
n |
% |
(95% CI) |
||||||||||||
Prominent scarring or bruising |
32 |
40% |
(28%–50%) |
||||||||||||
Difficulty finding veins to inject into |
29 |
36% |
(25%–46%) |
||||||||||||
Benzodiazepine dependence |
16 |
20% |
(11%–28%) |
||||||||||||
Swelling of arm |
18 |
23% |
(13%–31%) |
||||||||||||
Swelling of hand |
11 |
14% |
(6%–21%) |
||||||||||||
Abscesses or infections from injecting |
10 |
13% |
(5%–19%) |
||||||||||||
Thrombosis/blood clots |
7 |
9% |
(2%–15%) |
||||||||||||
Other† |
19 |
23% |
(14%–32%) |
||||||||||||
* Injection-related problems were reported by similar proportions of injecting drug users who inject capules only. Only three participants reported injecting tablets only, of whom one had reported difficulty finding veins to inject into. † Other problems reported included overdose, swelling of legs, swelling of feet, skin ulcers, dirty “hit”, hospitalisation, contact with ambulance and contact with police. |
|||||||||||||||
Received 3 September 2003, accepted 22 July 2004
- Courtney L Breen1
- Louisa J Degenhardt2
- Amanda D Roxburgh3
- Raimondo B Bruno4
- Rebecca Jenkinson5
- 1 National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW.
- 2 School of Pharmacy and School of Psychology, University of Tasmania, Hobart, TAS.
- 3 Turning Point Alcohol and Drug Centre Inc., Fitzroy, VIC.
The study was funded by the (then) Commonwealth Department of Health and Ageing (CDHA). In addition to the authors, the following researchers and research institutions contributed to the information presented: Ms Anthea Duquemin, Ms Barbara Gray, Ms Linda Hipper and Ms Susan Vesperman, Department of Health and Community Services, NT; Ms Jane Fischer, Mr Stuart Kinner and Professor Jake Najman, Queensland Alcohol and Drug Research and Education Centre, University of Queensland; Ms Jackie Hallam and Associate Professor Stuart McLean, School of Pharmacy, University of Tasmania; and Mr Craig Fry, and Dr Peter Miller, Turning Point Alcohol and Drug Centre, Inc., VIC. The following individuals and organisations generously provided information or secondary data for this article: David Pearson, National Medicines Policy Section, CDHA, for background information; Kevin McGeechan (Health Communication Network) and Andrew Kemp (Medilinx), for GPRN data; Maxine Robinson, John Dudley, Peter Marlton, Julie Lindner and David Theodore, for both non-PBS and PBS prescription data (the Drug Utilisation Sub Committee’s (DUSC) Drug Utilisation Database, Pharmaceutical Benefits Branch, Health Access and Financing Division, CDHA. Dr Katherine Conigrave provided helpful comments on an early draft, and Professor Wayne Hall provided useful comments on a later draft of the manuscript. Stuart Gilmour provided patient statistical advice and conducted time-series analyses on our behalf. We thank the agencies and individuals that assisted with recruitment and interviewing of IDUs. We thank the IDUs who were willing to be interviewed.
None identified.
- 1. Darke S. The use of benzodiazepines among injecting drug users. Drug Alcohol Rev 1994; 13: 63-69.
- 2. Ross J, Darke S. The nature of benzodiazepine dependence among heroin users in Sydney, Australia. Addiction 2000; 95: 1785-1793.
- 3. Strang J, Griffiths P, Abbey J, Gossop M. Survey of injected benzodiazepines among drug users in Britain. BMJ 1994; 308: 1082.
- 4. Dupont RL. Abuse of benzodiazepines: the problems and the solutions. Am J Drug Alcohol Abuse 1998; 14(Suppl 1): 1-69.
- 5. Iguchi MY, Handelsman L, Bickel WK, Griffiths RR. Benzodiazepine and sedative use/abuse by methadone maintenance clients. Drug Alcohol Depend 1993; 32: 257-266.
- 6. Gelkopf M, Bleich A, Hayward R, et al. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1 year prospective study in an Israeli clinic. Drug Alcohol Depend 1999; 55: 63-68.
- 7. Klee H, Fluagier J, Hayes C, et al. AIDS-related risk behaviour, polydrug use and temazepam. Br J Addiction 1990; 85: 1125-1132.
- 8. Ross J, Darke S, Hall W. Benzodiazepine use among heroin users in Sydney: patterns of use, availability and procurement. Drug Alcohol Rev 1996; 15: 237-243.
- 9. Gutierrez-Cedollada J, De La Torre R, Ortuño J, et al. Psychotropic drug consumption and other factors associated with heroin overdose. Drug Alcohol Depend 1994; 35: 169-174.
- 10. Eddey DP, Westcott MJ. ‘The needle and the damage done’: Intra-arterial temazepam. Emerg Med 2000; 12: 248-252.
- 11. Fry C, Bruno R. Recent trends in benzodiazepine use by injecting drug users in Victoria and Tasmania. Drug Alcohol Rev 2002; 21: 363-367.
- 12. Topp L, Kaye S, Bruno R, et al. Australian Drug Trends 2001. Findings from the Illicit Drug Reporting System (IDRS). Sydney: National Drug and Alcohol Research Centre, University of New South Wales; 2002. (NDARC Monograph Number 48.)
- 13. Commonwealth Department of Health and Ageing. Australian Statistics on Medicines, 1999–2000. Canberra: Commonwealth Department of Health and Ageing; 2003.
- 14. Sayer GP, McGeechan K, Kemp A, et al. The General Practice Research Network: the capabilities of an electronic patient management system for longitudinal patient data. Pharmacoepidemiol Drug Saf 2003; 12: 483-489.
- 15. Box G, Jenkins G. Time series analysis: forecasting and control. San Francisco: Holden-Day; 1976.
- 16. SPSS for Windows [computer program]. Version 11.0. Chicago, Ill: SPSS Inc, 1996.
- 17. Feeney G, Gibbs H. Digit loss following misuse of temazepam. Med J Aust 2002; 176: 380. <MJA full text>
- 18. Darke S, Ross J, Teesson M, Lynskey M. Health service utilisation and benzodiazepine use among heroin users: findings from the Australian Treatment Outcome Study (ATOS). Addiction 2003; 98: 1129-1135.





Abstract
Objective: To assess the effect of a restriction on publicly subsidised temazepam 10 mg capsules upon the injection of benzodiazepines by injecting drug users (IDUs).
Design and participants: Cross-sectional study of regular IDUs targeting periods before and after the policy change. Analysis of prescription data, including time-series analysis.
Setting: Drug services in the capital cities of New South Wales, Victoria, Tasmania, Queensland and the Northern Territory.
Main outcome measures: Changes in prescriptions and patterns of benzodiazepine use; harms associated with benzodiazepine use.
Results: There was a decrease in temazepam 10 mg capsule prescriptions and a corresponding increase in temazepam 10 mg tablet prescriptions after the policy change. IDU survey data suggested that IDUs continued to inject benzodiazepines and temazepam capsules. The frequency of the injection of capsules after the restriction appeared similar to that before the policy change. There was no change in the frequency of injection of tablets. Most IDUs reported obtaining their benzodiazepines from doctors, with substantial proportions obtaining capsules even after the restriction. About half the IDUs reported purchasing benzodiazepines on the street. Most IDUs who injected benzodiazepines reported injection-related problems.
Conclusion: Limiting the prescribing of temazepam capsules may have reduced their injection by some IDUs, but additional strategies are needed to reduce the misuse among this group. These may include further restriction of capsule preparations, continued education of doctors and IDUs, and the examination of prescribing practices of individual doctors.