Ambulatory stabilisation programs for adults with newly diagnosed diabetes have been in existence for over 50 years,1 and those for children for almost 25 years.2 Several outpatient stabilisation programs have been established in paediatric centres in North America, Europe and the Middle East, but children with newly diagnosed type 1 diabetes in Australia are traditionally admitted to hospital for several days to receive initial medical care and diabetes education. Most children are medically stable once treatment has commenced, but remain in hospital until the detailed education program has been completed. This process may result in significant disruption to families.3
In November 2000, we established a Diabetes Day Care Program (DDCP) at the Children’s Hospital at Westmead. We envisaged that it would be suitable for most children and teenagers presenting to our hospital with newly diagnosed type 1 diabetes. Ours was the first ambulatory stabilisation program in a paediatric diabetes centre in Australia. While studies evaluating ambulatory programs have shown that outpatient stabilisation of children and adolescents newly diagnosed with type 1 diabetes is safe, efficacious and cost-effective,4 there are few data on the psychosocial impact of such programs on families.5,6
Our aims were (i) to evaluate the benefits and adverse effects of the DDCP for children newly diagnosed with type 1 diabetes, and (ii) to compare length of hospital stay, diabetes knowledge, and metabolic and psychosocial outcomes over the first year after diagnosis in two cohorts, one diagnosed before and the other after implementing the DDCP.
Before the DDCP was introduced, children with newly diagnosed type 1 diabetes were admitted to hospital for 4–7 days for a detailed education program given by a diabetes educator and a dietitian. Families returned to a new-patient clinic within 3–6 weeks and then progressed to routine outpatient visits.
The DDCP is staffed by a multidisciplinary team consisting of diabetes educators, dietitians, social workers, endocrinologists and junior medical staff. The program consists of three phases:
Phase 1: Families attend the diabetes day care centre for 2–3 successive days, including weekends, during which they receive “survival skills” diabetes education. The family is given A guide for newly diagnosed diabetes7 and encouraged to purchase a comprehensive diabetes manual.8 An endocrinologist and a diabetes registrar or diabetes fellow are available 24 hours a day, and families are given clear instructions about when to contact the on-call team and when to return to the hospital.
Phase 2: Families attend three to four detailed “formal education” sessions. Phases 1 and 2 involve about 16 hours of education sessions in total.
Phase 3: Families attend an outpatient clinic at about 4–6 weeks after diagnosis, followed by routine 3-monthly outpatient visits.
The starting insulin dose is 0.3–0.5 units/kg per day, divided into two to four daily injections depending on age and individual suitability. In the first 2–4 weeks, families ring the diabetes educator daily for insulin doses until the doses have stabilised and they become confident with insulin adjustment.
The DDCP commenced in November 2000. Our study was conducted from March 2001 to October 2002. Two consecutively diagnosed cohorts were compared — one diagnosed in the 9 months before we introduced the DDCP (“pre-DDCP”) and the other diagnosed in the 9 months after its introduction (“post-DDCP”). Both cohorts completed the same questionnaire twice: once at the 6-month follow-up and again at the 12-month follow-up. Because of the timing of the study, not all families in the pre-DDCP cohort were sent the 6-month questionnaire. Patients were identified through our internal database and invited to participate in the study.
The questionnaire included four sections:
Parental diabetes knowledge was assessed using a modified version of the Test of Diabetes Knowledge.9 This was modified from the original 39-item multiple-choice tool to a 28-item test by removing items which, for our study population, were inappropriate or outdated.
The Parent Emotional Adjustment to Diabetes (PEAD) scale was adapted from the ATT (attitude) 39 scale and the ATT19 subscale.10,11 In the final analysis, 16 items were included based on reliability and factor analyses (coefficient α, 0.85). Lower scores on the PEAD scale indicate that parents perceive their child’s diabetes as having a greater emotional impact.
The Diabetes Responsibility and Conflict Scale12 is a 28-item questionnaire with two subscales designed to measure parental perceptions of responsibility, and the degree of conflict between parents and children with diabetes for 14 diabetes regimen activities. Parents completed both subscales.
Results are presented as mean (95% CI) for normally distributed data and median (range) for non-parametric data. We used SPSS for statistical analyses.14 Student’s t-test was used to compare groups with normally distributed data, and the Kruskal–Wallis test was used for non-parametric data. When appropriate, the level of significance was adjusted for multiple comparisons (Bonferroni correction).
There were 49 patients in the pre-DDCP cohort and 61 in the post-DDCP group. The post-DDCP cohort comprised 50 children (82%) who participated in the DDCP and 11 who were unsuitable for the DDCP.
The 6-month questionnaires were returned 7.8 (range, 6–9) months after diagnosis and the 12-month questionnaires were returned 14.6 (range, 12–16) months after diagnosis. The questionnaire response rates for the pre- and post-DDCP cohorts are shown in Box 1. There were no differences in demographic or baseline characteristics between respondents and non-respondents.
The baseline characteristics of the two groups showed no statistically significant differences in age, social disadvantage risk score, educational level of parents, and venous pH (Box 2). All patients diagnosed after the DDCP was introduced are included to enable analysis by intention to treat.
Ambulatory stabilisation of children with newly diagnosed type 1 diabetes produced medical outcomes in the first year after diagnosis comparable with those of children managed as inpatients. The time spent in hospital after diagnosis was reduced, without compromising knowledge and psychosocial outcomes, including coping skills, in the first year after diagnosis.
HbA1c levels and insulin requirements in the first year after diagnosis were similar in the pre-DDCP and post-DDCP cohorts, suggesting that metabolic control and the partial remission phase of type 1 diabetes were not affected by ambulatory stabilisation. Our data are similar to those reported in previous studies showing that ambulatory stabilisation does not have an adverse effect on glycaemic control,4 and may, in fact, have a beneficial effect.5
In the first month after diagnosis, children and families who receive outpatient education show similar psychosocial and cognitive outcomes to those managed as inpatients.6 Our study has extended these findings to 12 months after diagnosis (12-month questionnaire), giving greater weight to the argument that outpatient education and stabilisation of children with type 1 diabetes is no impediment to longer-term adjustment.
We found high levels of parental diabetes knowledge in both groups in our study, as did another study using the same scale.6 The high scores may reflect the fact that the questionnaires were completed at home with open access to diabetes resources, or that some items may be too easy to answer. In addition, the Test of Diabetes Knowledge measures theoretical rather than practical knowledge or problem-solving skills.
Mean PEAD scale scores suggested that, on average, parents in both groups experienced mild to moderate levels of diabetes-related emotional distress. At the time of diagnosis of type 1 diabetes in a child, families must absorb a lot of information, modify habits and family lifestyle, and abruptly take on increased responsibilities for treatment plans and management at home. We might have expected that parents put in this position without a “buffer zone” of hospitalisation would report a greater emotional impact of diabetes, but our data suggest there was no difference between the pre- and post-DDCP parent groups.
The main limitation of our study was its design. We were unable to conduct a randomised controlled trial, as we commenced our study after the DDCP program had been established. Hence, there may be systematic differences between the two cohorts. In addition, the time frame of our study meant that not all families in the pre-DDCP cohort were able to complete both questionnaires. There is only one previous randomised controlled trial of outpatient management of newly diagnosed type 1 diabetes in children and adolescents,15and further randomised controlled trials assessing longer-term outcomes are required.
Our study did not include assessment of each child’s psychosocial or cognitive outcomes. Research on paediatric populations documents hospitalisation as being perceived as traumatic.16 Therefore, depending on the age of the child, outpatient education, compared with education in hospital, may be less traumatic and less disruptive to school and social life. This is an important area for further research on children newly diagnosed with type 1 diabetes.
In conclusion, the DDCP is an example of an ambulatory paediatric service that
does not appear to compromise medical or psychosocial outcomes;
may be applicable to other chronic childhood illnesses; and
may be modified according to available resources.
However, longer-term assessment of psychosocial outcomes in children and their families using updated paediatric diabetes-specific tools is required.
1: Questionnaire response rates in the pre- and post-Diabetes Day Care Program (DDCP) cohorts
Questionnaire |
Pre-DDCP cohort (n = 49) |
Post-DDCP cohort |
|
||||||||
Eligible for DDCP (n = 50) |
Ineligible for DDCP (n = 11) |
||||||||||
6-month follow-up questionnaire |
22/24 (92%)* |
45/61 (74%) |
|||||||||
12-month follow-up questionnaire |
41/49 (84%) |
41/61 (67%) |
|||||||||
* Twenty-five families were not sent the 6-month follow-up questionnaire because of the timing of the study in relation to their diagnosis. |
|||||||||||
2: Baseline characteristics of the children in the pre- and post-Diabetes Day Care Program (DDCP) cohorts
Characteristic |
Pre-DDCP (n = 49) |
Post-DDCP (n = 61) |
P |
||||||||
Age (years) (median, range) |
8.76 (1.23–16.22) |
8.10 (1.09–15.89) |
0.39 |
||||||||
Social disadvantage risk score* (mean, 95% CI) |
0.47 (0.27–0.67) |
0.50 (0.31–0.69) |
0.83 |
||||||||
Education: no. (%) of parents with tertiary education |
Mother: 24/49 (49%) |
Mother: 33/61 (54%) |
0.59 |
||||||||
Father: 23/49 (47%) |
Father: 28/61 (46%) |
0.91 |
|||||||||
Venous pH at diagnosis (mean, 95% CI) |
7.32 (7.28–7.35) |
7.28 (7.25–7.32) |
0.20 |
||||||||
Length of hospital stay (days) (median, range) |
5.14 (2–10) |
1.70 (0–10) |
< 0.001 |
||||||||
* Valid range: − 5.21484 (least social advantage) to + 1.83241 (greatest social advantage).
3: Insulin requirement and glycohaemoglobin (HbA1c) level at 3, 6 and 12 months after diagnosis
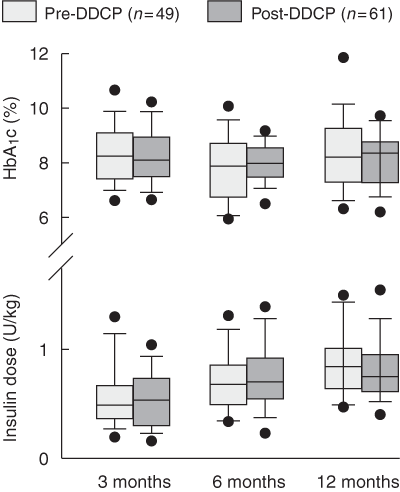
Error bars represent 95% CIs and data points represent outliers. DDCP = Diabetes Day Care Program.
4: Knowledge, emotional adjustment, responsibility and conflict scores in pre- and post-Diabetes Day Care Program (DDCP) cohorts. Except where otherwise indicated, data are mean (95% CI)
Questionnaire |
Time since diagnosis |
Pre-DDCP |
n* |
Post-DDCP |
n |
P† |
|||||
Test of Diabetes Knowledge (median [range] percentage correct answers) |
6 months |
96% (79%–100%) |
22 |
96% (75%–100%) |
45 |
0.78 |
|||||
12 months |
96% (75%–100%) |
40 |
96% (64%–100%) |
41 |
0.87 |
||||||
Parent Emotional Adjustment to Diabetes (valid range: 16 [greatest emotional impact] – 80 [least emotional impact]) |
6 months |
45 (41.2–48.8) |
22 |
39.9 (36.9–42.9) |
45 |
0.51 |
|||||
12 months |
43.2 (40.4–46.0) |
41 |
40.3 (37.4–43.2) |
41 |
0.50 |
||||||
Diabetes Responsibility and Conflict Scale |
|
|
|
|
|
|
|||||
Responsibility (valid range: 14 [least parental responsibility] – 70 [greatest parental responsibility]) |
6 months |
45.2 (38.1–52.3) |
22 |
49.7 (45.6–53.7) |
44 |
0.23 |
|||||
12 months |
45.5 (41.0–50.0) |
41 |
46.5 (41.9–51.2) |
41 |
0.74 |
||||||
Conflict (valid range: 14 [least parent–child conflict] – 70 [greatest parent–child conflict]) |
6 months |
22.5 (20.0–25.1) |
21 |
21.4 (19.1–23.7) |
41 |
0.58 |
|||||
12 months |
23.7 (21.0–26.3) |
36 |
21.4 (18.4–24.4) |
41 |
0.24 |
||||||
* The discrepancy in the number of pre-DDCP respondents to the questionnaires is because children of 25 families were diagnosed between 6 and 12 months before the study commenced and were only sent the 12-month questionnaire (21 responded). Some families did not complete all sections of the questionnaires.
† Bonferroni correction for multiple comparisons was used to determine significance at P < 0.025.
Received 7 August 2003, accepted 15 January 2004
- Shubha Srinivasan1
- Maria E Craig2
- Linda Beeney3
- Rachel Hayes4
- Nuala Harkin5
- Geoffrey R Ambler6
- Kim C Donaghue7
- Christopher T Cowell8
- Institute of Endocrinology and Diabetes, The Children’s Hospital at Westmead, Westmead, NSW.
We wish to acknowledge the diabetes team involved in the Diabetes Day Care Program during the study period (Mandy Crocker, Angela Middlehurst, Catherine Kay, Karen Jameson, Kristine Savage, Gillian Groves, Carolyn Judge, Samantha Clarke, Danielle Flachs, Melissa Loos and Kate McGuiness), Christine Fan for providing length of stay data, and Dr Neville Howard and Professor Martin Silink for giving us permission to contact their patients.
None identified.
- 1. Walker JB. Field work of a diabetic clinic. Lancet 1953; ii: 445-447.
- 2. Laron Z, Galatzer A, Amir S, et al. A multidisciplinary, comprehensive, ambulatory treatment scheme for diabetes mellitus in children. Diabetes Care 1979; 2: 342-348.
- 3. Schum TR. Effects of hospitalization derived from a family diary. Review of the literature. Clin Pediatr 1989; 28: 366-370.
- 4. Clar C, Waugh N, Thomas S. Routine hospital admission versus out-patient or home care in children at diagnosis of type 1 diabetes mellitus. Cochrane Database Syst Rev 2003; (3): CD004099.
- 5. Dougherty G, Schiffrin A, White D, et al. Home-based management can achieve intensification cost-effectively in type I diabetes. Pediatrics 1999; 103: 122-128.
- 6. Siminerio LM, Charron-Prochownik D, Banion C, Schreiner B. Comparing outpatient and inpatient diabetes education for newly diagnosed pediatric patients. Diabetes Educator 1999; 25: 895-906.
- 7. Diabetes Team, Children’s Hospital at Westmead. The Diabetes Day Care Program. A guide for newly diagnosed diabetes (pamphlet produced at the Children’s Hospital at Westmead), 2001.
- 8. Ambler G, Barron V, May C, et al. Caring for diabetes in children and adolescents, a parent’s manual. 2nd ed. Sydney: Combined Children’s Diabetes Services of NSW, 2001.
- 9. Johnson SB. Manual for the general information and problem-solving components of the test of knowledge. Gainesville, Fla: University of Florida,1979.
- 10. Dunn SM, Smartt HH, Beeney LJ, Turtle JR. Measurement of emotional adjustment in diabetic patients: validity and reliability of ATT39. Diabetes Care 1986; 9: 480-489.
- 11. Welch G, Beeney LJ, Dunn SM, Smith RBW. The development of the Diabetes Integration Scale: a psychometric study of the ATT39. Multivariate Exp Clin Res 1996; 11: 75-88.
- 12. Rubin R, Young-Hyman D, Peyrot M. Parent-child responsibility and conflict in diabetes care. Diabetes 1989; 38 Suppl 2: 28.
- 13. Vinson T. Unequal in life — the distribution of social disadvantage in Victoria and New South Wales. Victoria: The Ignatius Centre for Social Policy and Research, 1999.
- 14. SPSS [computer program], version 9. Chicago, Ill: SPSS Inc, 1999.
- 15. Dougherty GE, Soderstrom L, Schiffrin A. An economic evaluation of home care for children with newly diagnosed diabetes: results from a randomized controlled trial. Med Care 1998; 36: 586-598.
- 16. Boyd JR, Hunsberger M. Chronically ill children coping with repeated hospitalizations: their perceptions and suggested interventions. J Pediatr Nurs 1998; 13: 330-342.





Abstract
Objectives: (i) To evaluate the benefits and adverse effects of a Diabetes Day Care Program (DDCP); and (ii) to compare outcomes in two cohorts diagnosed before and after implementing the DDCP (“pre-DDCP” and “post-DDCP”).
Design: Outcomes from the pre-DDCP cohort were compared with those of the post-DDCP cohort.
Setting: The study was conducted from March 2001 to October 2002 at the Children’s Hospital at Westmead.
Participants: The pre-DDCP cohort comprised all children newly diagnosed with type 1 diabetes from March 2000 to November 2000 (n = 49). The post-DDCP cohort were those diagnosed from November 2000 to August 2001 (n = 61).
Main outcome measures: Length of stay, adverse events, insulin requirement and glycohaemoglobin (HbA1c) level over the first year after diagnosis were ascertained from medical records. Questionnaires to measure parents’ knowledge of diabetes, emotional adjustment to diabetes, and responsibility for and conflict over specific diabetes management tasks were completed by parents at 6-monthly intervals.
Results: Median length of hospital stay decreased from 5.14 days (range, 2–10) to 1.70 days (range, 0–10) (P < 0.001). There were no differences between the two cohorts in insulin requirement at 12 months (pre-DDCP: 0.9 U/kg [95% CI, 0.8–1.0]; post-DDCP: 0.8 U/kg [95% CI, 0.7–0.9]; P = 0.22), HbA1c level at 12 months (pre-DDCP: 8.4% [95% CI, 8.0%–8.9%]; post-DDCP: 8.2% [95% CI, 7.9%–8.5%]; P = 0.37) and adverse events over the first year after diagnosis. Both groups reported similar scores for the parental questionnaires.
Conclusions: Ambulatory stabilisation of children with type 1 diabetes provides similar metabolic outcomes for the child, and comparable levels of diabetes knowledge and similar psychosocial outcomes for the family, to inpatient stabilisation programs.