In the past decade, much has been learnt about the regeneration and repair of skeletal tissues. Regeneration involves slow replacement of tissues with identical tissue. It occurs readily in the embryo, hardly at all in neonates, and is never observed in adults. This may be because of the relatively high proportion of undifferentiated progenitor cells found in embryos and their scarcity in adults (1 in 10 000 mesenchymal cells in a newborn compared with 1 in 2 ×106 mesenchymal cells in an 80-year-old adult).1 In contrast, repair is a more rapid process and is probably designed for survival. It involves the usual inflammatory cell cascade, followed by matrix deposition and then a remodelling process which attempts, in part, to regenerate damaged tissues in the adult. A now extensive knowledge base of both repair and regeneration in “orthopaedic tissues” has enabled the development of orthopaedic tissue engineering.
Tissue engineering involves the use of cells coupled with biological or artificial matrices, or “scaffolds”, which guide the cells during tissue repair or regeneration. These cells can be “driven” by specific bioactive molecules, ex-vivo gene transfer and other physical factors to form neotissues in vitro for future reimplantation in vivo. Alternatively, the cells and special matrices, which can include bioactive molecules such as growth factors, can be combined in vivo to attempt to enhance tissue repair. An example is human articular cartilage repair using the patient’s own autologous chondrocytes retrieved at arthroscopy. These are expanded in vitro before reimplanting them into full-thickness articular cartilage defects covered with a sutured and fibrin-glued periosteal patch. Such articular cartilage repair has been shown to be clinically effective and durable up to 7 years.2
Here I will cover recent applications of tissue engineering to the repair of components of the knee joint, and compare this repair with normal tissues. I will also briefly address how tissue-engineering concepts have enhanced orthopaedic repair outcomes.
I will not describe the use of gene technology, as, to date, none of this technology has been applied in humans. However, there are many examples of the exciting use of genetic engineering in enhancing orthopaedic tissue repair in animals. Also, I will not discuss the use of embryonic stem cells.
In recent years the isolation of growth factors such as transforming growth factor-β3 and its analogues, such as the bone morphogenic proteins (BMPs) BMP-24 and BMP-7 (OP-1),5 has led to their being used clinically to enhance and accelerate bone repair, and also to replace bone. Bone induction to assist and enhance bone deposition and repair was first introduced by Marshall Urist in 1965,6 and led to the isolation of the BMPs, which could stimulate osteogenic precursor mesenchymal stem cells (MSCs) to form bone. Human cDNA BMP-7 (OP-1) was cloned in 1990. Recombinant human OP-1 (rhOP-1)7 followed, and was shown to induce bone formation in animals by stimulating osteogenic precursor MSCs.
In 2002, it was shown that injecting recombinant human OP-1 into a bone non-union site resulted in healing of the non-union after 30 months.8 OP-1 acts by recruiting adult osteogenic precursor MSCs to repair the bone defects (Box 1). In an Australian study of 163 consecutive patients, including 113 patients with long bone fracture and in whom non-union occurred, OP-1 was used at a mean of 23.2 months after injury, with a follow-up of 19 months. Clinical bone union was achieved in 70% of patients, and radiological bone union in 65%; 35% of these patients had previous failed bone autografts. No significant adverse reactions were attributed to the OP-1 implant. There was also a decreased incidence of osteomyelitis at the surgical site, and bone donor site pain was eliminated, as reflected in decreased use of postoperative analgesia.8
Intraoperative adult stem cell-based technologies are being developed to enhance repair of bone, especially in patients with delayed fractures or non-union of fractures.9,10 The basis of this work is that about 1 in 23 000 cells of adult bone marrow are osteogenic precursor cells. These cells can be separated from other adult haemopoietic stem cells by selective cell adsorption. This can be done in the operating theatre, making viable cell implants immediately available for surgical use. In theory, the use of adult osteogenic precursor stem cells in adequate numbers, combined with a suitable scaffold or matrix, may be better than using a conventional bone autograft. This is because the transplanted osteogenic stem cells can immediately begin to proliferate and lay down a bone neomatrix without the necessity of removing the “old matrix” present in conventional bone autografts. The use of an established canine spinal fusion model has partially confirmed the above hypothesis of the efficacy of an enriched bone matrix composite graft plus an enriched cellular composite.10 Such cell-based technologies may result in decreased use of conventional bone banks, which use “dead” bone to induce new bone formation in patients with loss of normal bone volume.
Researchers have recently replaced an avulsed thumb distal phalanx with a tissue-engineered bone construct.11 The avulsion resulted from a traumatic injury involving both dorsal thumb skin and the whole of the distal phalanx of the thumb. The dorsal thumb skin was reconstructed using a pedicular abdominal flap. The distal phalanx was replaced with autologous osteogenic precursor cells (that had been harvested from the distal radius periosteum during abdominal flap severance) and expanded in vitro for 9 weeks. At 10 months after the cell implant, only 5% of the implant was new bone, and at 28 months there was some proximal subluxation of the distal phalangeal implant.
This was the first clinical attempt at such whole-bone tissue engineering, and initially appears to have succeeded. The results are very encouraging, but there are no recent follow-up data as to how the patient’s new thumb is performing.
Attempts have been made to repair tendons and ligaments using type I collagen gels and MSCs. This approach has not yet been used clinically. Injecting antisense decorin oligonucleotides (a complex that blocks DNA transcription) into a repairing ligament segment has shown that large collagen fibrils responsible for the high tensile strength of tendons can be re-formed in the injected areas and the tensile strength of these ligaments also improved.12,13 However, this research is has not yet been transferred to the clinical setting. The ability to form large collagen fibrils in adults would represent a huge improvement in anterior cruciate ligament (ACL) reconstructive surgery and to the longevity and “tightness” of such knee ACL reconstructions.14
Articular cartilage is a unique avascular, aneural and alymphatic load-bearing live tissue which is supported by the underlying subchondral bone plate. It is unique in that the extracellular matrix is composed of a complex combination of type II collagen fibrils which are specifically arranged and have bonded to them very large water-retaining molecules called aggrecan molecules. This combination of molecules gives articular cartilage its unique ability to resist the repetitive compressive load-bearing necessary for the activities of daily life without undergoing premature wear.
Articular cartilage damage is common and does not normally self-repair. Often, young athletes and other patients are left with defects over 1 cm in diameter, experience symptoms and seek pain relief. Tissue engineering (autologous chondrocyte implantation [ACI]; see Box 2 and Box 3) has been used to demonstrate the possibility of repairing symptomatic full thickness hyaline articular cartilage defects in the knee joint and, more recently, in the ankle joint.2,15 In 100 patients with full-thickness, large (1.5–12.0 cm2) chondral defects,2 good to excellent results were found in the isolated femoral condyle (92%), multiple lesions (67%), osteochondritis dissecans (89%), patella (65%) and femoral condyle with ACL repair (75%). Second-look arthroscopy in 53 patients showed good tissue fill, good adherence to the subchondral bone and seamless integration with adjacent cartilage, and an arthroscopic indentation hardness close to that of normal cartilage. Histological analysis of 37 biopsy specimens showed a correlation between hyaline-like tissue and good to excellent clinical results. Complications were minimal, with graft failure of less than 10% and symptomatic graft hypertrophy in about 7%.2
In Australia, this technology was first used in Melbourne with encouraging results in 16 patients reviewed at 9 months after autologous chondrocyte transplantation, showing reduction of pain and improved function.16
Similar excellent clinical results have also recently been reported from Melbourne for both knee-joint and talar dome lesions. In the knee study, 57 patients with 81 chondral knee lesions were followed up with both clinical subjective evaluation and magnetic resonance imaging as well as second-look arthroscopy and core-needle biopsy at 12 months. Significant improvement from before the procedure to 12 months after the procedure was found in both subjective and knee-function scores. Core-needle biopsies showed that 70% of lesions were hyaline or hyaline-like cartilage, with a “seamless” interface with the normal adjacent cartilage.17
In Perth the efficacy of cartilage tissue engineering for repair of articular cartilage defects has also been confirmed.18
There are other technologies that have been used to repair articular cartilage, such as microfracture of the subchondral bone plate and also mosaicplasty (where osteochondral plugs are removed from normal cartilage and press-fitted into areas where there is full-thickness, symptomatic loss of articular cartilage). Findings of recent well-designed studies suggest that both techniques are less efficacious than autologous chondrocyte implantation.19,20
Knee-joint meniscal tissue engineering is just commencing, as it is now widely understood that the knee joint menisci play a very important role in load-sparing and sharing, thus protecting the underlying femoral and tibial articular cartilage. Complete and partial meniscal loss caused by traumatic damage is well known to lead to premature articular chondrocyte cell death and osteoarthritis, probably as a result of repeated impact overloading. Hence, the replacement and repair of knee-joint menisci is now recognised as being vital for long-term articular chondrocyte health. Long-term studies of meniscal allografting have been encouraging in terms of tibio-femoral pain relief. Recent conclusions and recommendations for arthroscopic allograft meniscal transplantation is that the technique is realistic, causes minimal morbidity and should be reserved for selected symptomatic young patients. The long-term failure rates are not defined, but all will probably fail with time. In a study using 40 cryopreserved allografts, an overall failure rate of 22% at 7-year follow-up has been reported.21,22 Recently, a collagen meniscal implant has been accredited in Australia and North America for use in attempted repair of meniscal defects and tears rather than debridement.23
2: Illustration of autologous chondrocyte implantation (ACI) technology, based on Brittberg et al15
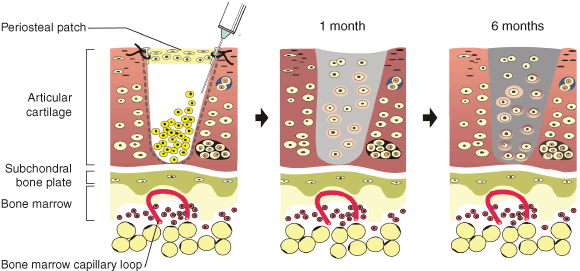
The articular cartilage defect is debrided (dashed line at margins of articular cartilage defect), which may kill adjacent chondrocytes down to the calcified layer of articular cartilage (white line at base of defect). A periosteal patch with the “cambium” layer of osteoprogenitor cells facing down into the defect is carefully sutured onto the top of defect (end to side) with about 1 mm interrupted 6/0 sutures and sealed with fibrin glue to ensure the chondral–periosteal compartment is “watertight”. Chondrocytes, which were retrieved at arthroscopy 3 weeks previously and proliferated at high density (1 × 106 cells/mL in vitro), are then injected carefully into the “contained” defect at a cell density similar to that of the native cartilage from which the cells were intially obtained. These cells settle, adhere and proliferate, and also lay down new cartilage .
3: Autologous chondrocyte implantation (ACI) to treat a large osteochondral defect in a young man
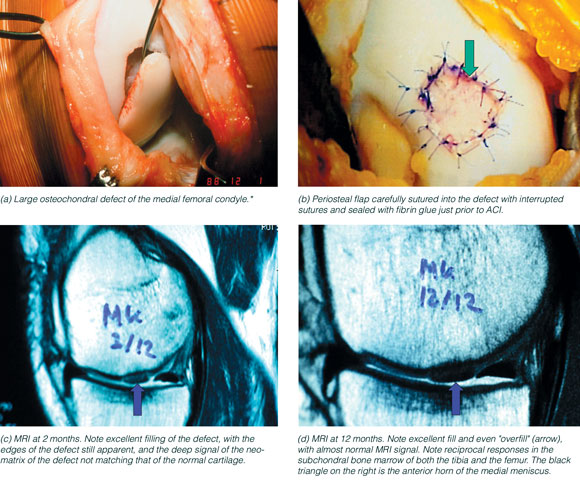
MRI = magnetic resonance imaging. * This injury is ideal for ACI using two periosteal membranes to separate the bone and chondral compartments. Clinical results of replacing such large articular osteochondral defects with pins and plugs have proved disappointing, probably because it is impossible for surgeons to obtain exact congruence of the fragment and normal articular cartilage to within the about 100 μm required for normal chondrocyte function with intermittent loading. Image (a) courtesy of Mr O Deacon, Orthopaedic Surgeon, Melbourne, and images (b), (c) and (d) courtesy of Mr I Henderson, Orthopaedic Surgeon and Director of Orthopaedic Surgery, St Vincent’s and Mercy Private hospitals, Melbourne. |
|||||||||||
- Barry W Oakes1
- Department of Anatomy and Cell Biology, Monash University, Clayton, VIC.
The author provides scientific advice to Mercy Tissue Engineering Ltd, for which a fee is received.
- 1. Haynesworth SE, Goldberg VM, Caplan AI. Diminution of the number of mesenchyme stem cells as a cause for skeletal ageing. In: Buckwalter JA, Goldberg VM, Woo SL-Y, editors. Musculoskeletal soft-tissue ageing: impact on mobility. Rosemont, Ill: American Academy of Orthopaedic Surgeons, 1994: 79-87.
- 2. Peterson L, Minas T, Brittberg M, et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop 2000; 374: 212-234.
- 3. Massague J. The transforming growth factor-β family. Ann Rev Cell Biol 1990; 6: 597-641.
- 4. Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenic protein gene family in bone formation and repair. Clin Orthop 1998; 346: 26-37.
- 5. Sampath TK, Coughlin JE, Whetsone RM, et al. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-β family. J Biol Chem 1990; 265: 13198-13205.
- 6. Urist MR. Bone formation by autoinduction. Science 1965; 150: 893-899.
- 7. Sampath TK, Maliakal JC, Hauschka PV, et al. Recombinant human osteogenic protein-1 (hOP_1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem 1992; 267: 20352-20362.
- 8. Giltaij LR, Shimmin AS, Friedlander G. Osteogenic protein-1 (OP-1) in the repair of bone defects and fractures of long bones: clinical experience. In: Vukicevic S, Sampath K, editors. Bone morphogenic proteins. From laboratory to clinical practice. Progess in Inflammation Research Series. Basel: Birkhauser Verlag, 2002: 193-205.
- 9. Muschler G, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop 2002; 395: 66-80.
- 10. Muschler GF, Nitto H, Matsukura Y, et al. Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop 2003; 407: 102-118.
- 11. Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med 2001; 344: 1511-1514.
- 12. Butler D, Awad HA. Perspectives on cell and collagen composites for tendon repair. Clin Orthop 1999; 367 Suppl: S234-S332.
- 13. Frank C, Shrive N, Hiraoka H, et al. Optimization of the biology of soft tissue repair. J Sci Med Sport 1999; 2: 190-210.
- 14. Oakes BW. Tissue healing and repair: tendons and ligaments. In: Frontera WR, editor. Rehabilitation of sports injuries. Scientific basis. The Encylopaedia of Sports Medicine. An IOC Medical Commission Publication. London: Blackwell Publishing, 2003: 56-98.
- 15. Brittberg M, Lindhal A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994; 331: 889-895.
- 16. Hart J, Paddle-Ledinek J. Treatment of articular cartilage defects in the knee with autologous chondrocyte grafting. Proceedings of the International Cartilage Repair Society 2nd symposium. Boston: ICRS,1998.
- 17. Henderson I, Tuy B, Connell D, et al. Clinical results and correlation with MRI findings of autologous chondrocyte implantation (ACI) with a minimum 12 months prospective follow up. J Bone Joint Surg 2003; 85: 1060-1066.
- 18. Wood D, Zhen M, Robertson B. An Australian experience of ACI and MACI. In: Bentley G, editor. Current developments in autologous chondrocyte transplantation. Round Table Series #77. London: The Royal Society of Medicine Press Ltd, 2003: 7-16.
- 19. Bentley G, Biant C, Carrington RJW, et al. A prospective, randomized comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg 2003; 85B: 223-230.
- 20. Knutsen G, Engbretson L, Ludvigsen TC, et al. Autologous chondrocyte implantation versus microfracture. A prospective randomized Norwegian multicentred-trial. Toronto: Proceedings of the Fourth International Cartilage Repair Society, 2002.
- 21. Noyes F. Meniscus function: repair and restoration. Meniscal allografts: indications, technique, results. Instructional Lecture Number 311. American Academy of Orthopedic Surgeons 70th Annual Meeting; 2003. Feb 7; New Orleans. Rosemont, Ill: AAOS, 2003.
- 22. Harner CD. Meniscal allografts: indications and techniques. Proceedings of ISAKOS Congress; March 10-14, Auckland, New Zealand, 2003; 1.19-1.22.
- 23. De Haven K. Collagen Meniscal implant 2003 update. Proceedings of ISAKOS Congress, March 10-14, Auckland, New Zealand, 2003; 1.14-1.18.






Abstract
Tissue engineering involves the use of cells (either adult, mesenchymal or embryonic stem cells) coupled with biological or artificial matrices or scaffolds which guide the cells during repair or regeneration of the tissue.
Recently discovered and isolated growth factors can promote either adult or stem-cell growth and differentiation along selected pathways to re-form and repair skeletal tissues in adults.
Bone repair enhancement and replacement is now possible with the use of tissue-engineering technologies.
It is now possible to repair articular cartilage using the patient’s own articular chondrocytes retrieved during arthroscopy, and expanded in vitro. Clinical results of this technique are very satisfactory.