Aboriginal Australians experience a higher risk of cardiovascular death than the general Australian population.1 They also have higher and increasing rates of diabetes.2,3 Diabetes undisputedly increases the risk of coronary heart disease (CHD).4-8 In the Western world, women are at a much lower risk of dying of heart disease than men.9 Among men and women without diabetes, the male to female CHD mortality ratio is consistently around two. However, it has been suggested that this “female advantage” is eliminated by the presence of diabetes.6,10 Several meta-analyses have reported that the impact of diabetes on the risk of CHD is greater for women than men.9,11,12 However, the interpretation of the sex differences in the impact of diabetes remains controversial.12 Kanaya et al reported that the excess risk of CHD outcomes in women with diabetes is mediated by well established modifiable cardiac risk factors.12
There are no prospective data available on CHD incidence in the presence of diabetes in Aboriginal populations. Our study aimed to (i) determine the incidence rate of CHD in Aboriginal people with type 2 diabetes; and (ii) compare the impact of diabetes on CHD risk in Aboriginal women and men.
From 1992 to 1995, a community-wide renal disease screening program, involving 897 adults aged 20–74 years, was conducted in a remote Northern Territory Aboriginal community. For our study, eight adults were excluded because their hospital records showed pre-existing CHD events. Thus, 889 people, representing over 80% of the community’s adult population, were included in our analysis.
We identified CHD events from hospital and death records using the codes of the International classification of diseases (ICD-9-CM codes 410–414, and ICD-10-AM codes I20–I25). Only first incidents (fatal or non-fatal) were included in the analysis. All participants were followed up to 31 May 2003. For those who reached a CHD event endpoint during follow-up, their follow-up period was the time from the date of their initial screening visit to the date of the first CHD event. Those who did not reach an endpoint were “censored” at 31 May 2003.
Baseline measurements of established CHD risk factors, such as age, sex, smoking status, diabetes, total and high-density lipoprotein (HDL) cholesterol levels, body mass index (BMI), and blood pressure, have been described elsewhere.2
There were two groups of participants with diabetes.
The “baseline diabetes” group included participants who, at the baseline examination, had self-reported “known diabetes” or were being treated for diabetes; or had been hospitalised previously with diabetes, with the diagnosis confirmed from hospital records; or were newly diagnosed at the baseline examination using a fasting serum glucose value and a 2-hour glucose tolerance test.13 Participants with either overnight fasting serum glucose or 2-hour post-load glucose levels over World Health Organization thresholds13 were considered as having diabetes.
The “new diabetes” group included participants who were not known to have diabetes at the baseline examination, but were diagnosed by examining hospital records during the follow-up period.
The “non-diabetes” group included all remaining participants who had not been diagnosed as having diabetes as at 31 May 2003.
The data were partitioned into four age groups: < 35, 35–44.9, 45–54.9 and 55 + years. For individuals who were in more than two different age groups during the follow-up period, the total follow-up period was subdivided into two or more person-time records, as described by Clayton and Hills.14 Incidence rates were estimated according to diabetes status for each age group, using the number of first-ever CHD events divided by the person-years of follow-up. Rate ratios and their 95% confidence intervals were estimated, with participants without diabetes as the reference group. We used the Cox proportional hazards model, adjusting for potential confounding factors of age, sex, smoking, blood pressure, and HDL cholesterol and total cholesterol levels. The proportion of CHD in the study population attributable to diabetes was expressed as the population-attributable fraction. Cumulative CHD incidence proportion during the follow-up period was calculated by the Kaplan–Meier method. All analyses were performed using Stata 8.0.15
The 889 participants were followed for 7129 person-years, with a median follow-up period of 8.7 years. Of these, 123 (13.8%) were identified as having diabetes at the baseline examination. A higher proportion of women (17%) had diabetes than men (11%) (risk ratio, 1.5; 95% CI, 1.1–2.1).
Another 65 participants (7.3%) were hospitalised and diagnosed as having diabetes during the follow-up period.
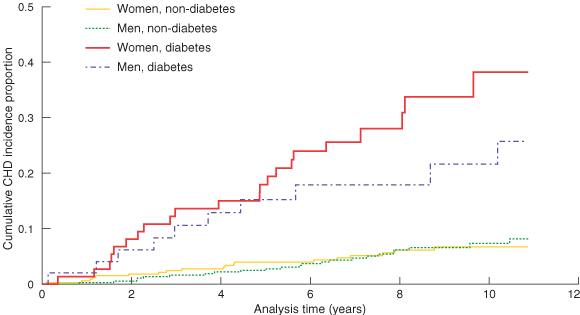
Eighty-nine participants had one or more CHD events, including 16 whose first-ever CHD event was fatal. Incidence rates of CHD were 7.3 (95% CI, 5.4–9.9), 28.3 (95% CI, 17.1–47.0) and 37.5 (95% CI, 26.5–53.0) per 1000 person-years for the non-diabetes, new diabetes and baseline diabetes groups, respectively. We compared the CHD incidence rates of the baseline diabetes and non-diabetes groups. As shown in Box 1, both women and men with baseline diabetes had higher blood pressure, total cholesterol and triglyceride levels, BMI and waist circumference, and lower HDL cholesterol levels. In Box 2, Kaplan–Meier curves show estimates of the proportion of participants with coronary heart disease events or diagnoses during the study period. Participants with baseline diabetes had a higher CHD risk than those without diabetes. The difference was much greater in women. Women with diabetes not only had a higher CHD risk than women without diabetes, but also appeared to develop, over time, a higher risk than men with diabetes.
Age- and sex-specific incidence rates were estimated. Although the 95% CIs were wide (because the sample sizes were small), higher point estimates of CHD rates among participants with diabetes were found in all age and sex strata (Box 3).
After adjusting for age, the CHD rate for women with diabetes was 4.2 (95% CI, 2.2–8.0) times that for women without diabetes, while the CHD rate for men with diabetes was 1.7 (95% CI, 0.8–3.9) times that for men without diabetes. Adjusting for age, smoking status, alcohol consumption, BMI, blood pressure, HDL cholesterol and total cholesterol levels, the rate ratios were 3.7 (95% CI, 1.6–8.9) and 1.4 (95% CI, 0.4–4.1) for women and men, respectively.
The population-attributable risk fraction (ie, the proportion of CHD events in the study population attributable to the presence of diabetes) in women was 45% (95% CI, 21%–62%) after adjusting for age, and 48% (95% CI, 14%–69%) after adjusting for age, smoking status, alcohol consumption, blood pressure, HDL cholesterol and total cholesterol levels. For men, the population-attributable risk fraction was 11% (95% CI, − 11% to 32%) after adjusting for age, and 3% (95% CI, − 20% to 22%) after adjusting for age, smoking, alcohol consumption, blood pressure, BMI, HDL cholesterol and total cholesterol levels.
CHD risk did not differ significantly between female and male participants without diabetes, although women had a slightly lower risk than men. Among those with diabetes, women had a higher risk than men, with an adjusted rate ratio of 1.8 (95% CI, 0.6–5.4). However, using combined data from both sexes, an interaction term between diabetes and sex was found to be not statistically significant using a Cox proportional hazards model, with adjustment of age, smoking, alcohol consumption, blood pressure, BMI, HDL cholesterol and total cholesterol levels.
Our study shows that diabetes in Aboriginal people entails a high risk of CHD. Women with diabetes experienced about four times the CHD risk of women without diabetes, while the corresponding value for men was 1.4 times.
Although the definitions of diabetes and CHD events may differ from study to study, the finding that diabetes has a greater impact in women than men is consistent with reported studies in other populations.4,9,11,12 A higher relative risk of CHD in women with diabetes has been reported in the Framingham study,5,7 the Atherosclerosis Risk in Communities (ARIC) study,4,16 and other studies, as shown in meta-analyses.9,11,12 Hoy et al reported that, in Navajo Indians, the relative risk of CHD was higher in women than men with diabetes, but men had a significantly higher absolute risk than women.17 We found that Aboriginal women with diabetes not only had a higher absolute CHD risk than women without diabetes, but also a higher risk than men with diabetes, although this difference was not statistically significant. The “female protective effect” on CHD risk found in other populations9 does not appear to exist in Aboriginal people with diabetes. Women without diabetes had a similar CHD risk to men without diabetes. It is not clear why the “female protective effect” did not exist in this population. More importantly, our data suggest that for women with diabetes the risk of CHD may exceed the CHD risk in men with diabetes — a possible female disadvantage phenomenon.
Why might women with diabetes have a higher relative risk of CHD than men with diabetes? As well as suggestions that the observed differences in CHD risk between men and women with diabetes are mediated by traditional cardiac risk factors,12 a popular explanation relates to HDL cholesterol level. HDL cholesterol levels, which are inversely associated with CHD, are found to be lower in women than men with diabetes.18,19 In our study, women with diabetes had higher HDL levels and higher BMI values than men with diabetes. However, parallel differences existed between women without diabetes and men without diabetes. The higher risk among women than men with diabetes persisted after adjusting for BMI, HDL level and traditional confounding factors. Therefore, this explanation is not supported by our findings.
There are a number of other explanations, including differences between men and women in coagulation, in the patterns of obesity, and a possible role for hyperinsulinaemia.11 Barrett-Connor et al suggested that the sex difference in the independent contribution of diabetes to heart disease was largely explained by the higher survival rate of women without diabetes.10 This may not be the case in our study population, as, first, there was no difference in CHD risk between women and men without diabetes; and, second, women with diabetes had a higher absolute incidence of CHD than men with diabetes.
Although it is not clear why women with diabetes had a higher risk of CHD, Aboriginal women with diabetes may progress faster than men to the onset of CHD. This may reflect poorer management of diabetes in Aboriginal women. As the prevalence of diabetes risk is increasing in Aboriginal people,3 improving the management of diabetes in Aboriginal women is important to prevent CHD. A basic principle of prevention is that the intensity of risk-reduction therapy should be adjusted to a person’s absolute risk.20 The higher risk in women should be considered when deciding on aggressive treatment.
Some limitations of this study should be pointed out. First, the CHD events were identified through hospital and death records. Some minor CHD events not severe enough for hospitalisation may have been missed. Similarly, some participants in the non-diabetes group might have developed diabetes but were not hospitalised during the follow-up period. This might partly explain the low risk among men. Second, a CHD event was more likely to be identified if a patient had been hospitalised for other conditions such as diabetes, and vice versa. This may have overestimated the association between diabetes and CHD. Third, even though we took several confounding factors, such as age, sex, blood pressure, alcohol consumption, BMI, HDL and total cholesterol levels and smoking, into consideration, other confounding factors (eg, inflammation) remained. Fourth, because the sample size is relatively small, the extent of the increased risk may not be precise, as reflected in the wide 95% confidence intervals. The finding of increased CHD risk of women with diabetes relative to men with diabetes is inconclusive and needs to be confirmed in future research. Fifth, the study was conducted in a remote community. It remains to be verified whether the “female disadvantage” is a general phenomenon in other Australian Aboriginal populations.
1: Adjusted means (and 95% CIs)* of selected CHD risk factors according to diabetes status
|
Non-diabetes |
Baseline diabetes |
P |
||||||||
Women |
n = 330 |
n = 74 |
|
||||||||
Systolic BP (mmHg) |
116.3 (114.4–118.1) |
119.3 (115.4–123.3) |
0.180 |
||||||||
Diastolic BP (mmHg) |
70.7 (69.4–72.1) |
74.6 (71.7–77.4) |
0.020 |
||||||||
Total cholesterol (mmol/L) |
4.3 (4.2–4.5) |
4.9 (4.6–5.2) |
0.0002 |
||||||||
HDL cholesterol (mmol/L) |
1.1 (1.0–1.1) |
1.0 (0.9–1.0) |
0.030 |
||||||||
Body mass index (kg/m2) |
23.7 (23.1–24.4) |
26.1 (24.7–27.5) |
0.0027 |
||||||||
Waist circumference (cm) |
89.1 (87.6–90.6) |
97.7 (94.4–100.9) |
< 0.001 |
||||||||
Men |
n = 371 |
n = 49 |
|
||||||||
Systolic BP (mmHg) |
124.4 (122.8–126.0) |
133.6 (128.9–138.4) |
0.0004 |
||||||||
Diastolic BP (mmHg) |
76.7 (75.3–78.0) |
81.2 (77.3–85.1) |
0.032 |
||||||||
Total cholesterol (mmol/L) |
4.9 (4.8–5.0) |
5.3 (4.9–5.7) |
0.045 |
||||||||
HDL cholesterol (mmol/L) |
1.2 (1.1–1.2) |
1.1 (1.0–1.2) |
0.037 |
||||||||
Body mass index (kg/m2) |
22.6 (22.2–23.1) |
26.3 (25.0–27.6) |
< 0.001 |
||||||||
Waist circumference (cm) |
85.5 (84.1–86.6) |
95.3 (91.7–99.0) |
< 0.001 |
||||||||
* Adjusted for age. BP = blood pressure. HDL = high-density lipoprotein. |
|||||||||||
2: Kaplan–Meier curves showing estimates of the proportion of the participants with coronary heart disease (CHD), by sex and diabetes status
3: CHD incidence rates (per 1000 person-years), by diabetes status
|
Participants without diabetes |
Participants with diabetes |
|||||||||
Age (years) |
No. with first CHD event |
Person-years |
CHD rate |
No. with first CHD event |
Person-years |
CHD rate |
|||||
Women |
|
|
|
|
|
||||||
<35 |
3 |
1285 |
2.3 (0.8–7.2) |
1 |
92 |
10.9 (1.5–77.6) |
|||||
35–44 |
4 |
698 |
5.7 (2.2–15.3) |
8 |
142 |
56.2 (28.1–112.0) |
|||||
45–54 |
1 |
376 |
2.7 (0.4–18.9) |
7 |
175 |
40.5 (19.1–84.0) |
|||||
55+ |
11 |
284 |
38.7 (21.4–69.9) |
6 |
92 |
65.2 (29.3–145.0) |
|||||
Men |
|
|
|
|
|
||||||
<35 |
5 |
1798 |
2.8 (1.2–6.7) |
1 |
48 |
20.7 (2.9–147.0) |
|||||
35–44 |
8 |
877 |
9.1 (4.6–18.2) |
5 |
82 |
12.2 (1.7–86.6) |
|||||
45–54 |
7 |
260 |
26.9 (12.8–56.5) |
3 |
147 |
34.0 (14.2–81.7) |
|||||
55+ |
3 |
169 |
17.7 (5.7–55.0) |
3 |
76 |
39.7 (12.8–123.0) |
|||||
- Zhiqiang Wang1
- Wendy E Hoy2
- Department of Medicine, School of Medicine – Central Clinical Division, University of Queensland, Herston, QLD.
We especially thank the Aboriginal people who participated in this study and the Aboriginal community for its support. The baseline data were collected by the Renal Research Team at the Menzies School of Health Research. This project was funded by the National Health and Medical Research Council (NHMRC) of Australia.
None identified.
- 1. Mathur S, Gajanayake I. Surveillance of cardiovascular mortality in Australia 1985-1996. Canberra: Australian Institute of Health and Welfare, 1998.
- 2. Wang Z, Hoy W. Hypertension, dyslipidaemia, body mass index, diabetes and smoking status in Aboriginal Australians in a remote community. Ethn Dis 2003; 13: 324-330.
- 3. McDermott R, Rowley KG, Lee AJ, et al. Increase in prevalence of obesity and diabetes and decrease in plasma cholesterol in a central Australian Aboriginal community. Med J Aust 2000; 172: 480-484. <MJA full text>
- 4. Folsom AR, Chambless LE, Duncan BB, et al. Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes Care 2003; 26: 2777-2784.
- 5. Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation 1979; 59: 8-13.
- 6. Wilson PW. Diabetes mellitus and coronary heart disease. Am J Kidney Dis 1998; 32: S89-S100.
- 7. Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J 1985; 110: 1100-1107.
- 8. Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev 1987; 3: 463-524.
- 9. Orchard TJ. The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med 1996; 28: 323-333.
- 10. Barrett-Connor EL, Cohn BA, Wingard DL, et al. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991; 265: 627-631.
- 11. Lee WL, Cheung AM, Cape D, et al. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care 2000; 23: 962-968.
- 12. Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002; 162: 1737-1745.
- 13. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes melitus. Geneva WHO: Department of Noncommunicable Disease Surveillance, 1999.
- 14. Clayton D, Hills M. Statistical models in epidemiology. Oxford: Oxford University Press, 1993: 53-62.
- 15. StataCorp. Stata Statistical Software, Release 8.0. College Station. Tex: Stata Corporation, 2003.
- 16. Folsom AR, Szklo M, Stevens J, et al. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 1997; 20: 935-942.
- 17. Hoy W, Light A, Megill D. Cardiovascular disease in Navajo Indians with type 2 diabetes. Public Health Rep 1995; 110: 87-94.
- 18. Goldschmid MG, Barrett-Connor E, Edelstein SL, et al. Dyslipidemia and ischemic heart disease mortality among men and women with diabetes. Circulation 1994; 89: 991-997.
- 19. Walden CE, Knopp RH, Wahl PW, et al. Sex differences in the effect of diabetes mellitus on lipoprotein triglyceride and cholesterol concentrations. N Engl J Med 1984; 311: 953-959.
- 20. Executive summary of the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA 2001; 285: 2486-2497.





Abstract
Objectives: To determine the incidence rate of coronary heart disease (CHD) in Australian Aboriginal people with type 2 diabetes, and to compare the impact of diabetes on CHD risk in Aboriginal women and men.
Design: Cohort study.
Setting: A remote Aboriginal community in the Northern Territory.
Participants: 889 Aboriginal people aged 20–74 years followed up to 31 May 2003 after baseline examination in 1992–1995.
Main outcome measures: Incidence rates of CHD (estimated for 123 participants with diabetes at baseline and 701 “non-diabetes” participants); rate ratios for diabetes risk (95% CI), with “non-diabetes” participants as the reference group.
Results: Participants with diabetes at baseline had a higher rate of CHD (37.5 per 1000 person-years) than those without diabetes (7.3 per 1000 person-years). Adjustment for multiple CHD risk factors, such as age, smoking, alcohol consumption, systolic blood pressure, body mass index, high-density lipoprotein cholesterol and total cholesterol levels, resulted in a CHD rate ratio for women of 3.7 (95% CI, 1.6–8.9) (comparing women with diabetes with those without) and a CHD rate ratio for men of 1.4 (95% CI, 0.4–4.1) (comparing men with diabetes with those without).
Conclusions: Aboriginal women with diabetes experienced a significantly higher risk of CHD than women without diabetes. Although the difference was not statistically significant, women with diabetes had a higher CHD risk than men with diabetes.