In Australia, sources such as the Monthly Index of Medical Specialties (MIMS) are commonly consulted for approved product information (PI) on prescription medicines. Unfortunately, both in Australia1 and overseas,2-4 many PIs lack information concerning use in children. This is not a new issue. Indeed, it was more than 30 years ago that Shirkey first referred to paediatric patients as “therapeutic orphans”,5 highlighting the widespread use of disclaimers and absence of guidance regarding drug use in children.
Dose information is of utmost importance in treating children, who have differing pharmacokinetic and pharmacodynamic profiles.6 In Australia, an audit of the 1994 MIMS showed that PIs for 72% of prescription medicines either provided no information about paediatric use or contained a partial or general disclaimer about use in children.1 Methodological details for this audit are scant, and no formal studies in Australia have quantified the amount and type of paediatric dosing information provided in PIs.
In addition to dose information, clinicians require medicines to be in forms that are suitable for administering to children of different ages. For some medicines, although paediatric dosing information is provided, there is no commercially available paediatric dosage formulation.7,8 The proportion of medicines in this category is unknown.
Using MIMS drug references, we investigated the extent to which information is currently available on paediatric dosing, the nature of this information, and the availability of paediatric dosage forms in Australia.
We reviewed the PIs for all prescription medicines contained in the 1999 Australian MIMS annual 9 for information on paediatric use. We defined paediatric patients as children less than 12 years of age.6 To bring the 1999 data up to date, we used cumulative lists from MIMS bimonthly issues (December 1998/January 1999 to August/September 2001) to identify new products, revised PIs, deleted and reinstated products and presentations, and scheduling changes. New or revised PIs were reviewed using E-MIMS.10
In collecting data on dosing information, we initially chose age groups based on physiological characteristics: neonate (< 1 month), infant (1 month to < 2 years), child (2 years to < 12 years), adolescent (12 years to < 18 years) and adult (≥ 18 years).11 We later subdivided the infant group (1 month to < 3 months, 3 months to < 2 years) and the child group (2 years to < 6 years, 6 years to < 12 years), as dosing information contained in MIMS was often categorised in this manner.
Dosing information within the PI was assigned to one of six categories (Box 1) for each age group. Dosing information for a specific age group was considered “inadequate” if there was no suggestion for use of the product in the age group; disclaimers such as “safety and efficacy not established” were given; the product was not approved for use in the age group; dosing recommendations were non-specific or incomplete; or usage in the age group was suggested but no dosing information was given.
When dosing information did not fit exactly into the age group, allocation was made to the next closest age group. For example, the PI for Codalgin Forte tablets provides specific dosing information for patients ≥ 7 years. In this case, specific dosing information was allocated to patients aged 12–18 years and ≥ 18 years. Where generic equivalent products were available and the PIs of such products were incomplete, the PI from the innovator brand was referred to for information on paediatric dosing.
An independent researcher validated our categorisation system by reviewing a random sample of 100 PIs using the method described above. The researcher was blinded to the original categorisation. Generation of the random sample for validation was undertaken using MINITAB software.12 Descriptive statistical analysis was carried out using Systat software.13
A total of 1497 PIs from 21 therapeutic classes were reviewed. About 12% (181) of PIs contained dosing information that did not fit exactly into our defined age groups and thus had to be allocated to the next closest age group. These PIs were included in our data analysis.
Of the 133 medicines with generic equivalent products, there were inconsistencies in dosing information for 12.8% (17) of medicines. For example, paediatric dosing information was given for only four of the five brands of frusemide 40 mg tablets, although all five brands are bioequivalent.14 Other medicines with inconsistent dosing information included amitriptyline, clonazepam, clomiphene, phenoxymethylpenicillin, amoxycillin, cephalexin and tamoxifen. The PIs for medicines with inconsistencies in dosing information were included in our data analysis.
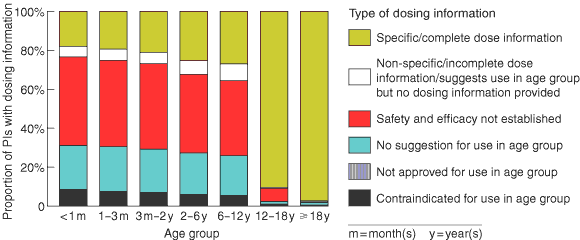
Box 2 shows the type and extent of dosing information available for different age groups. About 18% of PIs contained specific dosing information for neonates, and the proportion of PIs giving this information rose gradually with age group to around 27% in children aged 6–12 years. There was then a marked jump in the amount of specific dosing information available for adolescents (90.5% of PIs) and adults (97.3% of PIs).
About 0.5% of PIs contraindicated the use of a medicine in adults, and 2.2% provided inadequate dosing information for adults.
For products not contraindicated for use in neonates, 80.5% of PIs gave inadequate dosing information. The proportion of PIs with inadequate dosing information decreased with increase in age group: 1–3 months (79.1%); 3 months–2 years (77.5%); 2–6 years (73.2%); and 6–12 years (71.6%).
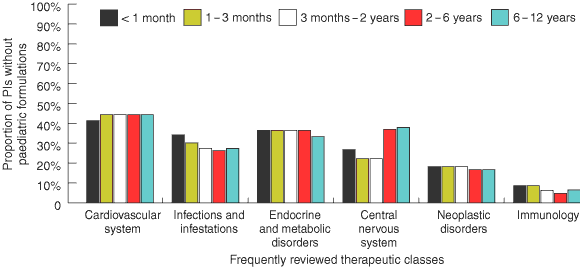
The most frequently reviewed therapeutic class was medicines for the cardiovascular system, with about 11% of PIs giving specific dosing information for paediatric patients. The therapeutic classes that contained the highest number of PIs with specific paediatric dosing information were medicines for immunology (37%–68% of PIs) and infections/infestations (35%–52%) (Box 3).
A substantial proportion of PIs that gave specific dosing information for paediatric patients did not have a paediatric dosage form for the medicine (age group [% without a paediatric dosage form]): < 1 month (26.5%); 1–3 months (25.1%); 3 months–2 years (23.3%); 2–6 years (21.9%); and 6–12 years (24.0%).
The availability of paediatric dosage forms for medicines varied according to therapeutic class. Among the 11% of PIs for cardiovascular system medicines that provided specific paediatric dosing recommendations, over 40% did not have appropriate dosage forms (Box 4). In the case of propranolol, for example, specific dosing information was given for treating children, but only tablets were available. For the immunology class medicines, a relatively small proportion of PIs (4.7%–8.6%) with specific paediatric dosing information were without a suitable paediatric dosage form (Box 4). Medicines in this class that were not available in paediatric dosage forms included Typh-Vax (available only as capsules) and three generic equivalents of azathioprine (available only as tablets).
Our study showed that up to 81% of PIs for medicines that are available in Australia and not contraindicated for use in children give inadequate paediatric dosing information. This figure is comparable to those found in US studies (78%–81%),2,3 but slightly higher than the 72% estimated by the Australian Drug Evaluation Committee.1 Our study shows that there has been little improvement in dosing information for children since Shirkey first coined the term “therapeutic orphan”.5
The lack of adequate paediatric dosing information is not evenly spread across therapeutic classes: for example, although cardiovascular system medicines were the most frequently reviewed therapeutic class, only 11% of PIs for these medicines contained specific paediatric dosing information. Paediatric information was also very inadequate for medicines relating to the central nervous system and endocrine disorders. Of the most commonly reviewed therapeutic classes, medicines for immunology and infections/infestations had the highest proportion of PIs with specific paediatric dosing information — a not unexpected finding, given that vaccines and antibiotics are frequently prescribed for children.
The reasons for the continued lack of dosing information for children are unclear. This issue has been previously raised in Australia, most recently in a 1997 report of an Australian Drug Evaluation Committee working party.1 Companies seeking registration of medicines do not regard drug testing in children as an attractive, viable or profitable option,15 presumably because a plethora of ethical and logistical dilemmas is associated with clinical studies in children. Can invasive monitoring techniques that are of no direct benefit to the child’s current therapy really be justified? How many children with “the illness” need to be recruited to produce the required endpoint? What about adherence? And costs?
Inconsistencies in dosing information between generic equivalent products occurred in about 13% of medicines. Given that PIs are frequently used by clinicians for drug information, this presents a major problem, as inconsistent information could have a direct impact on patient treatment.
A medicine with dosing information for paediatric patients should be available in a suitable paediatric formulation.16 Our study revealed numerous instances in which this was not the case and also showed that the absence of paediatric dosage forms is not evenly distributed across therapeutic classes. It is alarming that 22%–27% of medicines for which specific paediatric dosing information is given are not available in a paediatric dosage form. The absence of paediatric dosage forms may lead to problems such as higher rates of medication errors resulting from dilution of adult dosage forms and lack of stability associated with liquid formulations that are prepared by pharmacists from solid-dose forms.
There have been attempts to rectify the lack of paediatric drug information and dosage forms. Legislative changes in the United States have included the FDA Modernisation Act, the “Pediatric Rule”, and the Best Pharmaceuticals for Children Act. In Europe, the European Medicines Evaluation Agency published its Note for guidance on clinical investigation of medicinal products in the paediatric population17 to encourage the pharmaceutical industry to perform appropriate testing of products likely to be of benefit to children. The Therapeutic Goods Administration (TGA) in Australia has adopted these guidelines, as well as a number of other mechanisms such as monetary incentives, priority attention to submissions, and allowing the use of published literature to support submissions to the TGA (www.health.gov.au/tga/docs/pdf/litbsubs.pdf) to encourage greater submission of data on paediatric patients.18
Regulatory authorities need to introduce a mandatory requirement that all medicines with a potential use in children have paediatric dosing information and are available in appropriate dosage forms. Strong strategic partnerships are needed between the pharmaceutical industry, regulatory authorities, academic institutions and practitioners to bring about the necessary changes.15,19 The support of consumers will also be critical.19 It will be important to assess the impact of these initiatives in a few years’ time. Until then, it is likely that paediatric patients will continue to be “therapeutic orphans”.
1: Categories used to allocate type of dosing information
Product can be used:
specific* dosing information present for one or more indications; or
complete† dosing information present for one or more indications.
Product can be used:
non-specific‡ dosing information present for one or more indications; or
incomplete§ dosing information present for one or more indications; or
use in age group suggested but no dosing information provided.
PI states or implies that safety and effectiveness of product not established (eg, “not recommended for use”, “limited experience with use”).
No suggestion that product can be used in age group.
PI states product not approved for use in age group.
PI states product contraindicated for use in age group.
PI = product information.
* Criteria used for paediatric patients: dose (expressed in dose/weight and/or dose/body surface area [BSA]) and dosing schedule present. Also allocate this category if dose¶ and dosing schedule present for: (a) creams, ointments, lotions, nasal solution or drops, inhalation devices or solutions, eardrops and ointments, eyedrops and ointments, scalp application, rectal preparations (eg, suppositories), powders (eg, nutritional formulas); or (b) medicines with stat dose(s) (eg, as part of immunisation schedule; some antidotes; some antivenoms).
† Criteria used for adults: dose¶ and dosing schedule present.
‡ Criteria used for paediatric patients: dose not expressed as dose/weight or dose/BSA, or dose (expressed as dose/weight or dose/BSA) provided but no dosing schedule, or vice versa.
§ Criteria used for adults: dose¶ provided but no dosing schedule, or vice versa.
¶ (Dose does not need to be expressed as dose/weight or dose/BSA.)
2: Type and extent of dosing information available in MIMS 9 for 1497 prescription medicines, by age group*
PI = product information.
* Products contraindicated for use in adults include palivizumab injection and ribavirin inhalation, and a range of diphtheria/pertussis/tetanus products. Medicines with PIs that do not suggest use of the product in adults, imply lack of safety and effectiveness for use, provide incomplete dosing information, or suggest use in adults but do not provide dosing information include medicines used exclusively in children, such as vaccines for Haemophilus influenzae type b, some vaccines for diphtheria/pertussis/tetanus, surfactant therapy for neonatal respiratory distress syndrome, and therapy to close a patent ductus arteriosus.
- Elaine Tan1
- Craig R Rayner2
- Colin B Chapman3
- Noel E Cranswick4
- 1 Department of Pharmacy Practice, Victorian College of Pharmacy, Monash University, Parkville, VIC.
- 2 Department of Clinical Pharmacology and the Australian Paediatric Pharmacology Research Unit, Royal Children’s Hospital, Parkville, VIC.
We gratefully acknowledge the assistance of Dr Kay Stewart and Professor Roger Nation in reviewing the manuscript. We would like to thank Ms Phyllis Lau for validating the categorisation system used in the data collection. Thank you MIMS Australia for supplying us with the MIMS database in ASCII format, and Sigma Company for their financial support towards Ms Tan’s doctoral scholarship.
None identified.
- 1. Report of the Working Party on the Registration of Drugs for Use in Children. Canberra: Australian Drug Evaluation Committee, 1997.
- 2. Wilson JT. Pragmatic assessment of medicines available for young children and pregnant or breast-feeding women. In: Morselli PL, Garattini S, Sereni F, editors. Basic and therapeutic aspects of perinatal pharmacology. New York: Raven Press, 1975: 411-421.
- 3. Gilman JT, Gal P. Pharmacokinetic and pharmacodynamic data collection in children and neonates: a quiet frontier. Clin Pharmacokinet 1992; 23: 1-9.
- 4. ‘t Jong GW, Eland IA, Stricker BH, et al. Information for paediatric use of medicines in a product information compendium. Paediatr Perinat Drug Ther 2001; 4: 148-151.
- 5. Shirkey H. Therapeutic orphans [editorial]. J Pediatr 1968; 72: 119-120.
- 6. Barker C, Nunn AJ, Turner S. Paediatrics. In: Walker R, Edwards C, editors. Clinical pharmacy and therapeutics. 3rd ed. Edinburgh: Churchill Livingstone, 2003: 111-126.
- 7. Christensen ML, Helms RA, Chesney RW. Is pediatric labeling really necessary? Pediatrics 1999; 104 (3 Pt 2): 593-597.
- 8. Nunn AJ. Making medicines that children can take. Arch Dis Child 2003; 88: 369-371.
- 9. MIMS Australia. 1999 MIMS annual. 23rd ed. Sydney: MediMedia Australia Pty Ltd, 1999.
- 10. E-MIMS [computer program]. Version 4.00.0500. Sydney: Vivendi Universal Health, August 2001.
- 11. Royal College of Paediatrics and Child Health and the Neonatal and Paediatric Pharmacists Group. Medicines for children. London: Royal College of Paediatrics and Child Health, 1999.
- 12. MINITAB [computer program]. Version 12.23. State College, Pa: Minitab Inc., 1999.
- 13. SYSTAT [computer program]. Version 10. Chicago, Ill: SPSS Inc., 2000.
- 14. Schedule of pharmaceutical benefits for approved pharmacists and medical practitioners, February 2003. Canberra: Commonwealth Department of Health and Ageing, 2003.
- 15. Conroy S, McIntyre J, Choonara I, et al. Drug trials in children: problems and the way forward. Br J Clin Pharmacol 2000; 49: 93-97.
- 16. Nordenberg T. Pediatric drug studies. Protecting pint-sized patients. FDA Consum 1999; 33: 23-28.
- 17. European Medicines Evaluation Agency. Note for guidance on clinical investigation of medicinal products in the paediatric population. London: EMEA, 1999. Available at: www.emea.eu.int/pdfs/human/ich/271199EN.pdf (accessed Jul 2003).
- 18. Therapeutic Goods Administration. Medicines for paediatric use. TGA News March 2002; Issue 37.
- 19. Collier J. Paediatric prescribing: using unlicensed drugs and medicines outside their licensed indications. Br J Clin Pharmacol 1999; 48: 5-8.






Abstract
Objectives: To review the approved product information (PI) of prescription medicines to determine the extent and nature of information available on paediatric dosing and the availability of paediatric dosage formulations in Australia.
Methods: The PIs for all prescription medicines listed in the Australian Monthly Index of Medical Specialties (MIMS) were reviewed. Dosing information for each PI was categorised according to age groupings. PIs claiming suitability for use in paediatric patients were reviewed for information on the availability of paediatric dosage forms.
Main outcome measures: Proportion of PIs providing paediatric dosing information; availability of dosage forms suitable for children.
Results: A total of 1497 PIs were reviewed. The proportions, for each age group, of PIs with inadequate paediatric dosing information were: < 1 month (80.5%), 1–3 months (79.1%), 3 months–2 years (77.5%), 2–6 years (73.2%), and 6–12 years (71.6%). The proportions, for each age group, of PIs that gave specific paediatric dosing information but did not provide a paediatric dosage form were: < 1 month (26.5%), 1–3 months (25.1%), 3 months–2 years (23.3%), 2–6 years (21.9%), and 6–12 years (24.0%).
Conclusions: The PIs for many prescription products listed in MIMS do not adequately detail paediatric doses. Many medicines for which specific paediatric dosing information is given are not available in dosage forms appropriate for children.