Self-reported prevalence of prostate-specific antigen testing in South Australia: a community study
Carole B Pinnock, David P Weller and Villis R Marshall
MJA 1998; 169: 25-28
For editorial comment, see McCredie & Cox
Abstract -
Introduction -
Methods -
Results -
Discussion -
Acknowledgements -
References -
Authors' details
-
-
©MJA1998
Abstract |
Objective: To determine the prevalence and other
characteristics of self-reported blood testing
(prostate-specific antigen [PSA]) for prostate cancer in the
community. Design: Interview-based prevalence study. Participants and setting: 695 men aged 40 years and over in the Spring 1996 South Australian Health Omnibus survey of a probability sample of 3011 households, weighted to reflect the age and sex distribution of the South Australian population. Outcome measures: Number of men who had a PSA test in the preceding 12 months; number of first tests; the person initiating and performing the test; knowledge of the next step if test result abnormal; number of men visiting doctor for lower urinary tract symptoms in the preceding 12 months. Results: 20.3% of participants reported having a PSA test in the preceding 12 months; 62.1% were first tests. Prevalence of testing was highest in the age group 70-79 years (35.8%). Most tests were initiated by the general practitioner (41.2%) and by patients (35.7%). Of those tested, 45.3% had inadequate knowledge of the next step. Visiting a doctor for urinary symptoms was significantly associated with PSA testing (P < 0.001): 47.7% of men who visited a doctor for urinary symptoms had been tested compared with only 17.4% of those who did not visit the doctor for this problem. Only age and visiting a doctor for urinary symptoms were significant independent predictors of having a PSA test. Conclusions: Investigation of lower urinary tract symptoms contributed substantially to PSA testing, and those tested did not adequately understand the consequences. Our findings suggest a need for a better framework for PSA testing in general practice, including all important elements of decision-making, such as evidence and patient preference, as well as the means to ensure adequate patient counselling before testing. |
Introduction |
Screening for prostate cancer remains a topic of widespread debate.
Recent systematic reviews have concluded that there is currently
insufficient evidence to establish an overall benefit of prostate
cancer screening, and major health agencies have issued conflicting
recommendations.1-3
Randomised controlled trials examining this question have begun in
the United States and Europe,4,5 but results will not be available
for some years.
Nevertheless, prostate-specific antigen (PSA) testing of asymptomatic men is thought to be common in Australia,6,7 and the recent doubling in recorded incidence of prostate cancer in this country8,9 has been attributed to PSA testing. Little is known about the circumstances of such testing -- whether it occurs in general practice or other specialties, whether men are truly asymptomatic, whether the test is initiated by the patient or the doctor, and how much information about the test is provided to the patient. Most authors agree that men seeking a test should be informed about the risks as well as the benefits of taking the test,10,11 and that individual preferences should be taken into account.12-15 A community survey of self-reported participation in PSA testing confirmed high rates in the community (25% of men 40 years and over with no history of prostate cancer) and an even higher rate of intention to test (53.9%).16 The strongest predictor of past testing was a visit to a doctor for urinary tract symptoms, and the strongest predictor of intention to test was perceived vulnerability to prostate cancer.16 This study was undertaken in 1996 to expand these findings by establishing the prevalence of self-reported prostate cancer testing (PSA testing) over the preceding 12 months, the incidence of PSA testing (first tests), who initiated the test, who performed it, the association with investigation for urinary symptoms, and whether participants had understood the immediate consequences of taking the test. |
Methods | |
Survey |
Questions were included in the Spring 1996 South Australian Health
Omnibus survey, a multiple-user household interview survey
undertaken on behalf of a number of healthcare organisations in South
Australia. The sampling method provides a probability sample of the
South Australian population.17,18 Participants were asked whether they had visited the doctor for troublesome urinary symptoms in the past 12 months (as in a previous survey17), ever been diagnosed with prostate cancer or had a blood test for prostate cancer (PSA test) in the previous 12 months. Those who had been tested were asked who had initiated the test, who performed it and what they understood to be the next step if the test were abnormal. Question alternatives were derived from a previous qualitative study (who initiated test)19 and expert clinical opinion (who performed it, next step). Questions were tested for comprehensibility and acceptability in 50 pilot interviews before the survey. |
Analysis |
Data were weighted by household size, age, sex and geographical
region to benchmarks derived from the estimated resident population
at 30 June 1995 (Australian Bureau of Statistics). Because of the
clustered nature of the sample, confidence limits were calculated
after allowing for a design effect of 1.1, calculated using the method
of Kish,20 which inflates
the standard error.
We analysed data for men aged 40 years or older who had not had a diagnosis of prostate cancer, using SPSS for Windows.21 Statistical significances were determined by Pearson c2 tests or Fisher's exact test, and logistic regression by the forced entry method (the contribution is evaluated after removal of effects of all other variables). The contribution of demographic and urinary symptom variables was examined in a logistic regression model to identify independent predictors of having a PSA test in the preceding 12 months. |
Results | |
Sample |
In unweighted numbers, from the sampling frame of 4081 households,
3011 interviews were conducted, giving a response rate of 73.8%. The
reasons for not participating were refusal (548), no contact could be
established (301), selected respondent away for duration of study
(108), illness/mental incapacity (65) and respondent unable to
speak English (48). Of the 3011 people interviewed, 695 were men 40
years or older and 642 of these had not had a diagnosis of prostate
cancer. The average age of these 642 men was 58.3 years (SE, 0.50 years;
range, 40-91 years).
After weighting, 6.0% (95% confidence interval [CI], 4.45%-7.86%) of men aged 40 years or more reported being diagnosed with prostate cancer. The weighted sample size of men aged 40 years and over with no reported diagnosis of prostate cancer was 716. |
PSA testing and demographic factors |
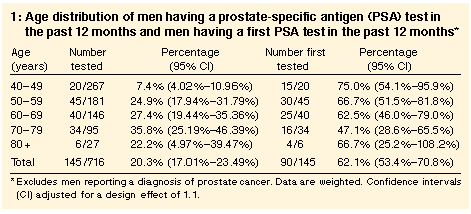
Overall, 20.3% of men older than 40 years reported having a blood test
for prostate cancer (PSA test) in the preceding 12 months (Table 1). This varied with age (chi-squared = 48.1; df = 4; P < 0.001),
with the highest proportion (35.8%) being in the age group 70-79
years. Nearly two-thirds of tests (62.1%) were first tests, and this
proportion was high across all age groups.
Men were slightly more likely to be tested if they lived in metropolitan (20.8%; 95% CI, 17.5%-24.1%) than in rural (19.0%; 95% CI, 15.8%-22.1%) areas, but the difference was not significant. Educational attainment was not associated with testing. Lifetime occupation also showed no clear trends (eg, drivers and plant operators had similar rates to managers and administrators), nor did marital status and country of birth. |
Lower urinary tract symptoms |
Of 715 men aged 40 years and over who had not had a diagnosis of prostate
cancer, 65 (9.1%) had visited a doctor for troublesome lower urinary
tract symptoms (LUTS) in the preceding 12 months (46 visited their
general practitioner). Of these 65 men, 31 (47.7%) had had a PSA test,
compared with only 113/650 (17.4%) of those who did not visit the
doctor for such a problem (P < 0.001, Fisher's exact test).
A first visit to a doctor for LUTS was significantly associated with a first PSA test (P = 0.018, Fisher's exact test): 15/17 (88.2%) men who had a first visit for LUTS in the previous 12 months also had a first test in that time, compared with only 6/14 (42.9%) men for whom it was not the first visit. |
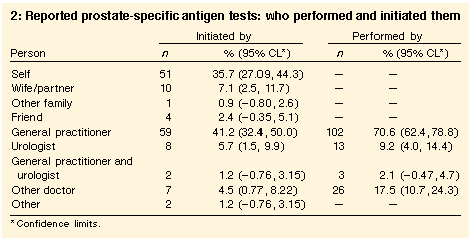
Who initiates and performs the test | Most testing (70.6%) was performed in general practice, and most tests were initiated by the general practitioner (Table 2). However, patients initiated 35.7% of tests. Tests performed by "other doctor" may reflect those done by pathology services, but could also have been included in other types of medical assessments. Doctors initiated 25 (80.6%) of the tests on the 31 men who visited for LUTS and were tested, but initiated only 51 (45.1%) of the tests on the 113 who were tested but had not visited a doctor for LUTS (chi-squared = 12.3; df = 1; P < 0.001). |
Knowledge of the "next step" | There was wide variability in understanding of the next step if the test were abnormal. The most frequent response was "do not know" (34.0%). Other responses were "operation on the prostate" (10.7%), "no further action" (0.6%), "referral to a specialist" (27.2%), "repeat the test" (6.3%), "biopsy of the prostate" (4.8%) and "other" (16.7%). The first three (do not know, operation on the prostate, no further action), totalling 45.3%, may be considered to reflect poor understanding. |
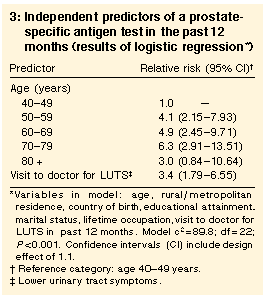
Independent predictors of testing | The logistic regression model (chi-squared = 89.8; df = 22; P < 0.001) included age, rural/metropolitan residence, country of birth, educational attainment, marital status, lifetime occupation, and doctor visit for LUTS in the past 12 months. Of these, only age and visit to a doctor for LUTS were significant independent predictors of having had a PSA test in the past 12 months (Table 3). |
Discussion |
We found a high level of self-reported PSA testing among men in South
Australia. Testing was associated with lower urinary tract symptoms
and was initiated by the patient or his GP. Nearly half the respondents
had an inadequate knowledge of the appropriate next step if the test
result were abnormal.
Our study was a community-based survey of self-reported testing. Such studies suffer from limitations in that individual recall of past events and comprehension of the research questions may vary. Nevertheless, representative community sampling makes it possible to derive estimates of incidence and prevalence, a benefit over general practice-based studies. The level of self-reported rates of PSA testing observed in this study is higher than that reported in New South Wales across all age groups.7 This may result from a real difference between South Australia and New South Wales or from differences in survey method. The sampling method differed between the two studies (probability sample weighted to reflect age and sex structure of South Australian population versus random telephone number selection from Sydney metropolitan white pages). Older men were under-represented in the New South Wales study compared with 1991 Census data. The interview method (face to face in South Australia versus telephone in New South Wales) may also have contributed to the differences. In our study, the number of men tested increased with age and peaked in the age group 70-79 years, with nearly half the tests in this age group being first tests. The choice to begin PSA testing at this age is of concern, as those least likely to benefit are men who can anticipate less than a decade of life.1 We found no association between PSA testing and socioeconomic factors such as education and occupation, although in the United States participation in prostate cancer screening is reported to be sensitive to socioeconomic factors.22,23 However, our result is consistent with other Australian data.7,16 It is also consistent with the view that testing is initiated more by doctors than patients in this community. There was only a small difference between rural and metropolitan testing rates, which suggests that access to services does not influence the number of men seeking or being offered testing. The role of LUTS in prompting testing for prostate cancer has been reported previously,7 and is important because of the high prevalence of such symptoms in the community. In a recent South Australian survey, 26.4% of men over 18 years reported experiencing troublesome urinary symptoms in the past 12 months, and 10.2% had visited a doctor for this reason. A similar number (8.6%) were substantially dissatisfied with their urinary function.17 The relative proportions of men and their doctor initiating prostate cancer testing have not been reported previously. We found that, in the absence of urinary symptoms, this is roughly equal. However, among those men who had visited a doctor for LUTS, 80% of tests were doctor-initiated. This strong association between doctor as test initiator and LUTS suggests that, while testing may be for case-finding or screening purposes in asymptomatic men, its use may be investigational for those with LUTS. We cannot know, from these and other data,7 whether other indicators for investigational PSA testing (such as abnormal or suspicious digital rectal examination, family history of prostate cancer, or complicated LUTS) were present, and therefore whether testing was appropriate in these cases. The current clinical guidelines for uncomplicated LUTS do not recommend testing for prostate cancer.24 The American College of Physicians maintains that, as no association between LUTS and prostate cancer has been demonstrated, testing in men both with and without LUTS consistent with benign prostatic enlargement constitutes screening.10 Current guidelines focus on when not to use the PSA test, but not when it is appropriate.24 There is no framework for doctors and patients which includes all the important elements of decision-making in this area, including evidence, patient preference and medicolegal issues. PSA testing in general practice is driven in part by concern of patients and in part for investigational purposes. In addition, there is anecdotal evidence that general practitioners are concerned that if a PSA test is not offered, and prostate cancer is later diagnosed, they may be seen as negligent. Clinical guidelines or other measures promoting the appropriate use of the PSA test in general practice need to give consideration to all of these factors. Undoubtedly, providing information about testing for prostate cancer is more complex than for breast cancer or cervical cancer screening. The finding that a large proportion of men tested did not understand the immediate consequences of testing is thus not surprising, but of concern. We cannot tell from these data whether information was not given, not understood, or not recalled. Nevertheless, it is reasonable to assume that if this very basic information is not understood effectively, then more complex information (such as the likelihood of a false positive result, side effects of a biopsy, effectiveness of treatment for early-stage prostate cancer, and the risks of impotence and incontinence resulting from radical surgery for localised cancer) would also not have been understood. Yet this has been suggested as basic information needed by a patient to understand the implications of a PSA test.11,14,15 We therefore see a need for closer examination of the exchange and uptake of information in consultations that result in a PSA test, and the provision of resources for GPs which aid effective counselling. Such resources may need to include longer consultations. |
Acknowledgements | The study was funded through a collaboration of Repatriation General Hospital, Daw Park, the Anti-Cancer Foundation of Australia, and the Department of Evidence Based Care and General Practice, Flinders University of South Australia, under the auspices of the Collaborative Centre for Prostate Health. |
References |
(Received 7 Nov 1997, accepted 7 May 1998) |
Authors' details
Division of Surgery, Repatriation General Hospital, Daw Park, SA.Carole B Pinnock, PhD, Principal Research Scientist.
Flinders University of South Australia, SA.
David P Weller, FRACGP, FAFPHM, Senior Lecturer, Department
of Evidence Based Care and General Practice;
Villis R
Marshall, MD, FRACS, Professor of Surgery, Department of
Surgery, Flinders Medical Centre, and Head, Division of Surgery,
Repatriation General Hospital, Daw Park, SA.
Reprints will not be available from the authors.
Correspondence:
Dr C R Pinnock, Division of Surgery, Repatriation General Hospital,
Daws Rd, Daw Park, SA 5041.
E-mail: spinncbATrgh.edu.au
-
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au>".
<URL: http://www.mja.com.au/>
Received 8 February 2026, accepted 8 February 2026
- Carole B Pinnock
- David P Weller
- Villis R Marshall