Prostate cancer in Western Australia: trends in incidence and mortality from 1985 to 1996
Timothy J Threlfall, Dallas R English and Ian L Rouse
MJA 1998; 169: 21-24
For editorial comment, see McCredie & Cox
Abstract -
Introduction -
Methods -
Results -
Discussion -
References -
Addendum -
Authors' details
-
-
©MJA1998
Abstract |
Objective: To measure trends in recorded incidence
and mortality rates of prostate cancer in Western Australia from 1985
to 1996 and to relate these to prostate-specific antigen (PSA)
testing for prostate cancer. Design: Descriptive study based on data from the Western Australian Cancer Registry, the Australian Bureau of Statistics and the Health Insurance Commission. Data: All newly diagnosed cases of prostate cancer and all deaths from prostate cancer in Western Australia from 1985 to 1996. Main outcome measures: Recorded incidences and mortality rates for prostate cancer. Results: After increasing steadily from 42 per 100 000 person-years in 1985 to 61 in 1992, the recorded incidence more than doubled to 134 per 100 000 person-years in 1994, then fell sharply to 87 in 1996. Among men aged 50 years or more, those aged 50-54 years had the largest annual increases: 14% (95% confidence interval [CI], 10%-19%) from 1985 to 1992 and 108% (95% CI, 84%-134%) from 1992 to 1994. They also had the smallest annual decline between 1994 and 1996 (8%; 95% CI, 1% increase to 16% decrease). The mortality rate showed no sudden increases or decreases. In men aged 60 years or older, the mortality rate increased annually by 2.9% (95% CI, 2%-4%) from 1985 to 1996. The number of Medicare reimbursements for PSA tests increased until May 1995, then fell. There was a significant correlation between the monthly number of PSA tests and new cases of prostate cancer (P < 0.01). Conclusions: Following a period of steady increase, the recorded incidence of prostate cancer increased dramatically in 1992 because of screening by PSA testing. From 1994, these incidence figures declined almost as sharply, partly because of reductions in testing. The mortality rate has not shown any systematic deviation from its long-term trend. |
Introduction |
In the early 1990s the recorded incidence of prostate cancer
increased dramatically in Australia,1,2 several years after a similar
increase in the United States.3-7 Testing for the disease among
asymptomatic men by measuring plasma prostate-specific antigen
(PSA) is believed to be responsible for the increases.1,3,4,7 We report here on trends in incidence figures and in the mortality rate for prostate cancer in Western Australia from 1985 to 1996, and their relationship to PSA testing. |
Methods | |
Data sources |
We obtained data on prostate cancer cases and deaths from the Western
Australian Cancer Registry, and population estimates from the
Australian Bureau of Statistics (ABS).8 We standardised rates to the World
Standard Population and calculated the risk of men developing
prostate cancer before the age of 75 years.9 Because mortality rates based on
coding of cause of death by the ABS were not available for 1996, we used
1996 rates from the Registry, which began coding causes of death in
1990; from 1990 until 1995, the two mortality rates were almost
identical.
Data relating to the number of PSA tests reimbursed by Medicare in Western Australia were obtained from the Health Insurance Commission in July 1997. |
Socioeconomic status |
To investigate any effect of socioeconomic status (SES) on recorded
incidence we used an index, derived from the 1991 census, in which each
census collection district (about 50 households) is assigned a
score.10 For Perth
patients, addresses at the time of diagnosis were mapped to
collection districts for the 1991 census using MapInfo.11 Geographical coordinate data
were provided by the Western Australian Valuer General's office and
the Department of Land Administration. The SES index was divided into
quarters of its distribution.
Because population data were available at the collection district level for census years only (ie, 1986 and 1991), we could not calculate SES-specific incidence. To determine whether any changes in numbers of cases by SES might be the result of different changes in population size in areas of different SES, we compared numbers of cases of prostate cancer and lung cancer. |
Trends in age-specific rates |
We used Poisson regression in EGRET12 to model age-specific recorded
incidences and mortality rates. Analyses of incidence were
restricted to men aged 50 years or older and analyses of mortality rate
to men aged 60 years or older because there were few events in younger
age groups. We analysed recorded incidence for each of the periods
January 1985 to December 1992, January 1992 to December 1994 and
January 1994 to December 1996. (Because the periods overlap, the
results were not independent.) A single analysis of mortality rate
was conducted. Likelihood ratio tests were used to obtain P
values.
We fitted age group as a categorical (ie, factored) variable and year of diagnosis as a continuous variable. The coefficient for year was exponentiated to give an annual percentage increase in the rate (eg, a coefficient of 0.35 when exponentiated is 1.42, equivalent to an annual increase of 42%). Age was added first, followed by the year of diagnosis, and then the interaction between the two. The interaction was fitted with age as a categorical variable and as a continuous variable, and the difference between these models was tested. Fitting the interaction with age as a categorical variable tests whether the secular trend was the same for all age groups; fitting it with age as a continuous variable tests whether there was a greater increase in younger men than in older men (or vice versa). In the analyses of incidence, P values for comparison of the two types of interaction were 0.17 for 1985-1992, 0.32 for 1992-1994 and 0.70 for 1994-1996. As there were no significant differences, the results reported for recorded incidence are from models in which the interaction involved age as a continuous variable. In all analyses, the final models provided good fits to the data -- the smallest P value for goodness-of-fit (for incidence in the period 1992-1994) was 0.09. |
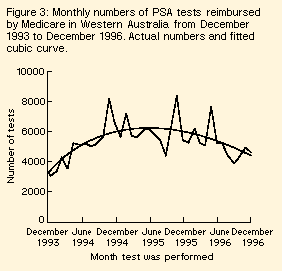
Prostate-specific antigen testing | Medicare began identifying PSA tests, together with prostatic acid phosphatase (PAP) tests, as a separate item during November 1993. Fewer than 1% of these tests would be PAP tests (Dr Glen Edwards, Chemical Pathologist, Western Diagnostic Pathology, personal communication). We plotted the number of tests reimbursed by Medicare each month from December 1993 to December 1996. To investigate the trends in the numbers of tests, we fitted a curve through the data. |
Results | |
Incidence |
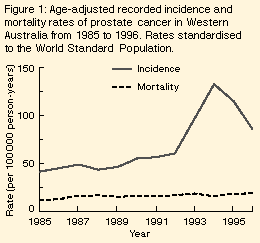
From 1985 until 1992, the age-adjusted recorded incidence increased
steadily from 42 to 61 per 100 000 person-years (Figure 1). In the next two years it more than
doubled to 134 per 100 000 person-years, but then fell almost as
sharply to 87 in 1996. The risk of prostate cancer before age 75 years
was one in 23 in 1985, one in six in 1994 and one in nine in 1996.
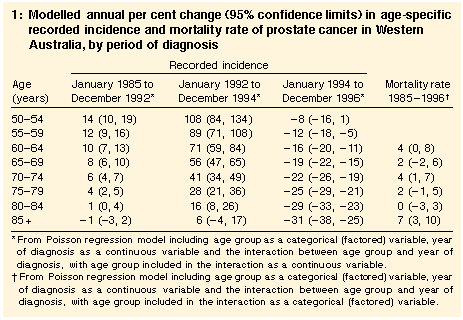
Age: In men aged over 50 years, the recorded incidence of prostate cancer increased annually by 5% (95% confidence interval [CI], 3%-6%) between 1985 and 1992 (trend, P < 0.001). The largest relative increases in recorded incidence between 1985 and 1992 occurred in the youngest men (Table 1; interaction between year and age, P < 0.001). The largest relative increases between 1992 and 1994 were also seen in the younger age groups (interaction between year and age, P < 0.001), and from 1994 to 1996 the decline was greatest in the oldest men (interaction between year and age, P < 0.001).
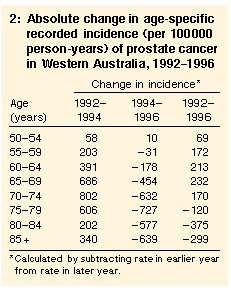
As a result of the different relative changes in different age groups, the differences in age-specific rates in 1996 were smaller than in earlier years. In 1985, the recorded incidence for men in the oldest age groups was close to 1000 times higher than for men aged 50-54 years, but by 1996 the relative difference was about 100-fold. We also examined the absolute changes in recorded incidence between 1992 and 1996 (Table 2). Between 1992 and 1994, the largest absolute increases were in men aged 65-79 years. Between 1994 and 1996, the largest absolute decreases were in men aged 70 years or older, so that between 1992 and 1996 the overall increases were greatest in men aged 60-69 years. The overall changes in men aged 55-59 years and 70-74 years were similar.
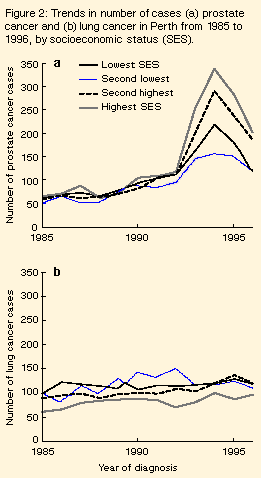
The mean age at diagnosis was 73 years in 1985, 74 years from 1986 until 1990, 73 years in 1991 and 1992, 72 years in 1993, 70 years in 1994 and 69 years thereafter. Place of residence: We examined age-standardised recorded incidence for prostate cancer separately for the Perth metropolitan region and the rest of Western Australia. Before 1992, the two rates were similar in all years. During the sudden rise and fall, these incidences were, respectively, 1992: 65 per 100 000 person-years (Perth), 51 per 100 000 person-years (non-metropolitan areas); 1993: 141 per 100 000 person-years, 107 per 100 000 person-years; 1994: 141 per 100 000 person-years, 110 per 100 000 person-years; 1996: 87 per 100 000 person-years, 84 per 100 000 person-years. Socioeconomic status: We mapped 94% of lung cancer and prostate cancer cases in the Perth metropolitan area to a 1991 census collection district, with no apparent trend over time in the proportion mapped. Before 1993, the numbers of prostate cancer cases in the four SES groups were similar (Figure 2). However, the increase in cases in 1993 and 1994 was greatest in areas of highest SES, and the largest declines in numbers of cases from 1994 to 1996 were also in these areas. Over the same period, there was no consistent change in the distribution of lung cancer cases by SES (Figure 2).
|
Mortality rate | The age-adjusted mortality rate from 1985 to 1996 showed no sudden increases or decreases (Figure 1). In men aged 60 years or older, the estimated annual increase from 1985 to 1996 was 2.9% (95% CI, 2%-4%; trend, P < 0.001). Adding quadratic (P = 0.78) or cubic (P = 0.92) terms for year did not improve the fit of the Poisson model. Furthermore, models using year as a continuous or a categorical variable fitted equally well (P = 0.14), indicating that year-to-year variations in the trend of the age-adjusted rates could be a result of chance alone. The increase differed across age groups (interaction between age as a categorical variable and year of death, P = 0.03), but the trends by age were inconsistent (Table 1; interaction between age as a continuous variable and year of death, P = 0.96). |
Prostate-specific antigen testing | On average, there were 5337 tests reimbursed each month in Western Australia. The numbers of tests initially increased before falling, although there was substantial monthly variation (Figure 3). A cubic curve fitted the data well (R2 = 0.46) and provided a better fit than a quadratic curve (P < 0.001) or a linear model (P < 0.001). The fitted maximum monthly number of tests occurred in May 1995. Spearman's rank correlation between the monthly number of PSA tests and new cases of prostate cancer was 0.48 (P < 0.01). |
Discussion |
After increasing steadily during the 1980s, the age-adjusted
recorded incidence of prostate cancer more than doubled between 1992
and 1994. By 1996, it had fallen to a level about 40% higher than that in
1992. In contrast, the mortality rate increased by about 3% per year,
with no sudden increases or decreases.
The youngest age groups showed the largest relative increases in recorded incidence up to 1994, and the oldest age groups showed the greatest decrease after 1994, causing a substantial compression of the range of age-specific recorded incidence in Western Australia. When the rates rose steeply in 1992, the absolute increases were greatest in men aged 65-79 years. However, men aged 70 years or older had the greatest absolute falls from 1994, so that, between 1992 and 1996, the largest absolute increases were in men aged 60-69 years. The increase appeared first in Perth and the peak was higher in Perth. However, by 1996, Perth and the rest of Western Australia had similar recorded incidences of prostate cancer. Within Perth, the changes were greatest in areas of high SES. Most observers have attributed the increases in recorded incidence of prostate cancer during the 1980s to improved case detection, particularly following transurethral resection of the prostate for benign prostatic hypertrophy.1,13 The sudden rise in incidence figures in about 1993 was observed in all Australian States.1 In the United States, similar dramatic increases were observed first in 1989.3 These increases are almost certainly the result of the introduction of screening by PSA testing.1,3 In Western Australia, free PSA testing during Prostate Awareness Week would have contributed to the increase. Each October from 1993, about 1100 men attended Prostate Awareness Week in Perth for PSA tests (Mr M D'Antuono, Biostatistician, Urological Research Centre, University of Western Australia, personal communication). These tests do not appear in the Medicare figures, although the peaks in Medicare-funded PSA tests in November 1994 and November 1995 might be the result of publicity surrounding Prostate Awareness Week. However, substantially fewer tests were performed at the screening venues than were reimbursed by Medicare each month in Western Australia. Thus, Prostate Awareness Week is unlikely to have greatly increased the recorded incidence in Western Australia. Rapid declines in recorded incidence, such as we found in Western Australia since 1994, have also been reported in some US States.4,6,7,14 Part of the Western Australian decrease is probably the result of reduced screening activity. The most likely explanation for the decrease in Medicare reimbursements that started in 1995 is a reduction in PSA testing for screening. In fact, the reduction in screening tests is probably greater than Figure 3 suggests, because PSA testing is also used for surveillance of men with prostate cancer. Therefore, its overall use will decline less rapidly than its use for screening. Widespread publicity in the media about the controversy surrounding screening for prostate cancer may have contributed to this decline, and the greater declines seen in the oldest men may be because of concerns that screening is unlikely to benefit those with a short expectation of life.15 Recorded incidence is expected to decrease even if screening activity remains constant. After the introduction of screening, recorded incidence increases because the time of diagnosis is advanced and some cases may be diagnosed that would not have become symptomatic. When all prevalent cases are detected, incidence figures will fall until new cancers develop, whereupon they will rise again. If screening detects only those cancers that would eventually have been diagnosed anyway, the recorded incidence will stabilise at its pre-screening level. Otherwise, it will stabilise at a higher level.16 Before 1980, the mortality rate from prostate cancer in Australia was stable for some time.17 Since then it has increased, but more slowly than recorded incidence, indicating that short-term survival, at least, has improved over time. Increasing diagnosis of disease with low potential for metastasis is one explanation for the discrepancy. What changes in mortality rate can we expect? Gann has argued that if screening is effective and covers enough of the population, the mortality rate should eventually decrease.16 It is too early for any effect of screening on mortality rate to be seen, and by the end of 1996 there had been no new trend in the mortality rate in Western Australia. Because of the effect of lead time (the time by which screening advances diagnosis), changes may not occur for some years. We have witnessed extraordinary changes in the recorded incidence of prostate cancer in Western Australia, and we have strong evidence that these changes are the result of medical practice rather than intrinsic changes in the incidence. Surveillance of incidence and mortality rates may help answer the question of whether screening has benefit, although more rigorous scientific evaluations are also needed. |
References |
|
Addendum | Since this article was submitted in September 1997, data on the recorded incidence and mortality rate of prostate cancer in the first eight months of 1997 have become available. The age-adjusted recorded incidence of prostate cancer was 64 per 100000 person-years, while the mortality rate was 16 per 100000 person-years. Thus, the recorded incidence was almost the same as in 1992, before the rapid rise and fall. In 1997, men aged 50-69 years had higher recorded incidences of prostate cancer than in 1992, but older men had lower recorded incidences. |
Authors' details
Health Department of Western Australia, East Perth, WA.Timothy J Threlfall, MB BS, MPH, Senior Medical Officer, Western Australian Cancer Registry;
Ian L Rouse, PhD, General Manager, Health Information Centre.
Department of Public Health, University of Western Australia,
Nedlands, WA.
Dallas R English, PhD, Senior Lecturer.
Reprints: Dr D R English, Department of Public Health, University
of Western Australia, Nedlands, WA 6907.
E-mail: dallasATdph.uwa.edu.au
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au>".
<URL: http://www.mja.com.au/>
Received 7 February 2026, accepted 7 February 2026
- Timothy J Threlfall
- Dallas R English
- Ian L Rouse