Reuse in sterile sites of single-use medical devices: how common is this in Australia?
Peter J Collignon, Elaine Graham and Dianne E Dreimanis
This article was published in the 6 May issue of The Medical Journal of Australia. Readers may print a single copy for personal use. No further reproduction or distribution of the articles in whole or in part should proceed without the permission of the publisher. For copyright permission, contact the Australasian Medical Publishing Company
See also articles by Brook and Woollard
Abstract - Introduction - Methods - Questionnaire - Definitions of cleaning/sterilising - Examination of devices - Results - Discussion - Reuse of medical devices - Cleaning and sterilisation - Recommendations for reuse - Cross-infection - Costs and cost benefit - Acknowledgements - References - Author's Details
Register to be notified of new articles by email - - ©MJA1996
Abstract
Objectives: To determine to what extent Australian hospitals reuse in sterile sites medical devices labelled "single use only"; to assess the adequacy of cleaning and sterilising procedures before reuse; and to estimate the possible incidence of cross-infection and the costs of not reusing these devices.Design: A self-administered questionnaire survey.
Setting: All Australian hospitals (419) with more than 45 beds and undertaking medical and surgical procedures.
Methods: Questionnaires were sent to hospital infection control practitioners in 1994 requesting information about reuse in sterile sites of single-use medical devices, the extent of reuse, the cleaning and sterilising processes involved, and the reasons for reuse.
Results: Responses were received from 168 hospitals (40%). Reuse occurred in 64 (38%), and another 33 hospitals had been reusing medical devices 12 months before our survey (i.e., 97/168 hospitals [58%] were either reusing them at the time of our survey or had been doing so 12 months previously). More large (> 300 beds) metropolitan public hospitals (9/14; 64%) reported reusing than did smaller (50/143; 41%) or private hospitals (15/47; 32%). At six of the 64 hospitals where reuse occurred, the process of cleaning and/or sterilisation of these devices was not satisfactory; from the information we received, both cleaning and sterilisation were satisfactory in only 38 hospitals (59%). Examination of the 14 most commonly reused devices showed that the structure of 13 of these may compromise cleaning (and therefore sterilisation). The main reason given for reuse was cost saving. Assuming a 2% prevalence of transmissible infections in blood, and an infection transmission risk of 1/500, we estimate that each year in Australia there may be 40 cases of cross-infection for every one million procedures performed with reused devices (0.004%).
Conclusions: Reuse of medical devices labelled "single use only" is common in Australian hospitals. Most devices appear to be unsuitable for reuse. Complete cessation of this practice of reusing single-use medical devices would stop potential cross-infection, but this would cost an estimated $2.5 million or more per case prevented.
MJA 1996; 164: 533-536
Introduction
Current guidelines generally recommend that medical devices labelled "single use only" should not be reused, but controversy arises because of their high cost and the belief that using them once only is wasteful as well as environmentally unsound.1,2 The United States Food and Drug Administration (FDA) maintains that there is a lack of data to support the general reuse of disposable devices.3 They consider that an institution or a practitioner reusing a disposable medical device should be able to demonstrate that it can be adequately cleaned and sterilised; that its physical characteristics or quality will not be adversely affected by the cleaning/sterilising process; and that the device will remain safe and effective for its intended use. In addition, the FDA believes that institutions or practitioners who reuse devices must bear full responsibility for their safety and effectiveness.3The Australian Confederation of Operating Room Nurses (ACORN),4 the Federation of Sterilising Research and Advisory Councils of Australia (FSRACA)5 and the Medical Industries Association of Australia (MIAA)6 strongly oppose the reuse of "single use only" medical devices. The Commonwealth, New South Wales, Queensland and Victorian health departments have all made recommendations against reuse,7-11 although most departments implicitly acknowledge that it occurs and they recommend guidelines for reuse similar to those of the FDA. Many refer to requirements of the Code of Good Manufacturing Practice (GMP) of the Therapeutic Goods Act 1989 (Cwlth).12 However, given the stringent quality control procedures, documentation and required sampling numbers of the GMP code, reuse of single-use devices in hospitals would not comply with GMP requirements.
Despite these directions and recommendations by regulatory authorities, there is anecdotal evidence that reuse occurs frequently in the belief that it saves money. However, for most devices the total costs of reusing have either not been calculated or do not take into account staff time and potential legal liabilities; reprocessing can at times be more expensive than purchasing replacements.
In view of the controversy surrounding this subject, and the lack of data, we aimed to determine both the extent of reuse of single-use medical devices in Australian hospitals and the perceived problems associated with this practice.
Methods In August 1994 we sent a questionnaire to all Australian hospitals with 45 beds or more where medical or surgical procedures were performed; 419 hospitals were identified (208 with < 100 beds, 147 with 100-300 beds, 49 with 300-600 beds, and 15 with > 600 beds).13 If after three months no reply had been received, individual responses were sought from those infection control practitioners in the non-responding institutions who were known personally to one of the authors. Replies were sought until the end of March 1995.
All respondents were given the option of returning the questionnaire anonymously and were assured that no identifiable data from their hospital would be released.
Questionnaire As well as information about frequency of reuse of single-use medical devices, the three-page questionnaire asked about: location of the hospital; the hospital size and type; specific details about cleaning and sterilisation methods for reused devices; and any change in hospital policy for reuse of single-use devices occurring in the previous 12 months, the reasons for any changes and whether the publicity from documented patient-to-patient transmission of HIV and hepatitis C in Australia14-16 had influenced any changes in practice. Respondents were also asked whether they were aware of any cross-infection resulting from reuse of single-use medical devices.
To questions about reuse of a list of medical devices that we considered had a high potential for reuse, respondents could answer "yes", "no" or "not used in this hospital". Details were requested from all areas of the hospital that may have been reusing. Specific areas and devices mentioned included: general surgery (diathermy pencils, other items); laparoscopic surgery (scissors, forceps, other items); gastroenterology -- endoscopic retrograde cholangiopancreatography (ERCP) (cannulas, stone-removing baskets, balloon dilators); gastroenterology -- colonoscopy (diathermy snares, sclerosing needles); gastroenterology -- endoscopy (upper gastrointestinal tract) (sclerosing needles, cytology brushes, other items); imaging (angiography catheters, other items); cardiology (cardiac catheters, pacing electrodes, other items); oncology (bone marrow trephine sets). Details of devices reused from other areas were also requested.
We sought information only on devices labelled "single use only" and used in sterile sites. Devices that were used in non-sterile sites (e.g., in gastroenterology procedures) were included if the device was used to breach a mucosal surface (e.g., a sclerosing needle or diathermy snare) and therefore entered a sterile site.
Definitions of cleaning/sterilising Physical cleaning may not remove all the biofilm, endotoxins and chemical residue from medical devices. If all organic material is not removed subsequent sterilisation may be compromised. Therefore, "satisfactory cleaning" required the use of ultrasonics and/or the use of a brush, detergent and a proteolytic enzyme. "Satisfactory sterilisation" required either autoclaving or "gassing" with ethylene oxide.
Because reusing these devices entails entry to sterile sites, glutaraldehyde or other chemical disinfectants were not regarded as achieving sterilisation. These agents do not achieve sterilisation without very long contact times and there are organisms resistant to these agents.
Examination of devices A visual examination was made of the structure of the 14 medical devices named above to assess potential problems with cleaning. Results
Reuse of medical devices
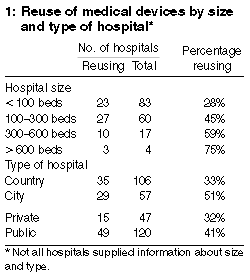 Of 419 hospitals sent the questionnaire, responses were received
from 168 (40%). Of these hospitals, 64 (38%) were reusing medical
devices. Thirty-three other hospitals were no longer reusing at the
time of our survey but had been doing so 12 months previously.
Twenty-one of the 64 hospitals reported that they had reduced the
number of devices being reused. Overall, 97 of 168 hospitals (58%)
either were reusing devices at the time of the survey or had been doing
so 12 months previously. Sixty-two of the reports were returned
anonymously. In 32 of these 62 hospitals (56%) reuse was occurring at
the time of the survey or 12 months previously. Large metropolitan
public hospitals (9/14; 64%) were more likely to reuse these devices
(Box 1).
Of 419 hospitals sent the questionnaire, responses were received
from 168 (40%). Of these hospitals, 64 (38%) were reusing medical
devices. Thirty-three other hospitals were no longer reusing at the
time of our survey but had been doing so 12 months previously.
Twenty-one of the 64 hospitals reported that they had reduced the
number of devices being reused. Overall, 97 of 168 hospitals (58%)
either were reusing devices at the time of the survey or had been doing
so 12 months previously. Sixty-two of the reports were returned
anonymously. In 32 of these 62 hospitals (56%) reuse was occurring at
the time of the survey or 12 months previously. Large metropolitan
public hospitals (9/14; 64%) were more likely to reuse these devices
(Box 1).
Cross-infection No institutions reported any cross-infection caused by reuse of single-use medical devices in sterile sites.
Cleaning and sterilising The cleaning and/or sterilisation processes in six hospitals were regarded as unsatisfactory. Twenty other hospitals reusing medical devices provided insufficient information on cleaning and sterilising. For 38 hospitals (59%) we considered that both cleaning and sterilisation were satisfactory.
Infection control committees One hundred and nineteen respondents commented that their infection control committees were concerned about the reuse of medical devices; 20 committees were not concerned (including eight committees at whose hospital reuse was occurring). In 26 hospitals the infection control committee had recommended that reuse of single-use devices should not occur, but reuse was still occurring. At 20 hospitals, infection control committees thought reuse appropriate in some instances, but no reuse was occurring in their hospitals. Altogether, 101 infection control committees had recommended against the reuse of single-use medical devices. Fifty-two hospital committees had stated that single-use devices could be used under defined circumstances. Fifteen hospitals did not supply information on this question.
Factors influencing reuse
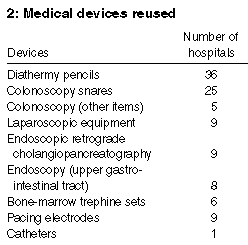 The main reason provided for reuse was cost saving. Other reasons
given were waste minimisation and environmental concerns. At 45
hospitals the adverse publicity surrounding documented
patient-to-patient transmission of HIV and hepatitis C in
Australia14-16 had
resulted in a decrease in both the types of devices reused and the
overall incidence of reuse. Twenty-two hospitals had discontinued
reuse altogether. The main medical devices that are being reused are
listed in Box 2.
The main reason provided for reuse was cost saving. Other reasons
given were waste minimisation and environmental concerns. At 45
hospitals the adverse publicity surrounding documented
patient-to-patient transmission of HIV and hepatitis C in
Australia14-16 had
resulted in a decrease in both the types of devices reused and the
overall incidence of reuse. Twenty-two hospitals had discontinued
reuse altogether. The main medical devices that are being reused are
listed in Box 2.
Examination of devices We were able to examine all 14 of the medical devices named in the questionnaire (see Methods); 13 of these probably could not be adequately cleaned because they had either a complex structure or small hollow lumens or crevices. Only pacemaker electrodes appeared to us to be readily cleanable.
Discussion
Reuse of medical devices
Reuse of single-use medical devices was a frequent practice in the
Australian hospitals responding to our questionnaire, and it was
more common in large metropolitan public hospitals. A Canadian study
(examining reuse of all single-use devices, not just those used in
sterile sites) also found more reuse in large hospitals.2 A recent draft report from the
National Health and Medical Research Council (NHMRC) indicated that
all 11 hospitals surveyed reused medical devices labelled as "single
use only".17 Medicolegal
implications and the media, however, appear to have had a major impact
on reuse of single-use medical devices. Recent adverse publicity
appears to have greatly influenced hospital practice, especially
over the past one to two years.
We believe our study underestimates the extent of reuse in Australia. The questionnaires were sent to infection control practitioners because of their knowledge and current understanding of the problem of reuse of single-use devices. We were surprised, however, that some institutions with well established infection control departments did not respond to our survey. This may be because of difficulty obtaining this information in large hospitals (with many different departments involved), or because of concerns about litigation and possible adverse publicity. Many questionnaires were returned with the proviso that the hospital's identity should remain strictly confidential.
Although large metropolitan teaching hospitals were under-represented among respondents, trends indicated that expensive devices (such as cardiac catheters and electrophysiology catheters) are more likely to be reused in these hospitals. Both reports in the literature and informal sources (e.g., media reports) suggest that these devices are frequently reused.18-20
Cleaning and sterilisation Provided an instrument can be satisfactorily cleaned and sterilised, it should not transmit infection and it does not matter that it is labelled single-use only. However, the physical characteristics (e.g., of the plastic) may not withstand cleaning and sterilising, making the device less safe to use. Any devices with hollow lumens or crevices, or those that cannot be disassembled for cleaning, are very difficult to clean and sterilise reliably, either by autoclaving or with ethylene oxide. In our study, a number of medical devices for which reuse appeared reasonable at first (e.g., single-use diathermy pencils) were found on closer examination to have spring-loaded buttons and crevices that would be difficult to adequately clean, particularly if contaminated with blood. Most disposable laparoscopic equipment appears to be very difficult to adequately clean, and most cannot be autoclaved because it is made of heat-sensitive materials. Ethylene oxide is less satisfactory than heat because it is less likely to penetrate hardened vegetative or protein matter (possibly harbouring microorganisms) remaining on inadequately cleaned medical devices. Other studies suggest that even when our requirements for "satisfactory cleaning" are followed, organic material frequently remains on these devices.21
Our study looked only at medical devices labelled "single use" and used in sterile body sites, but some of our reservations about devices whose design compromises cleaning are also valid for devices labelled reusable (e.g., laparoscopic equipment).
Recommendations for reuse
 The divergent views of infection control committees and hospital
practice found in our survey reinforce our view that it is difficult or
impossible for individual hospitals to assess in isolation whether
reuse of single-use medical devices is safe and cost efficient. Our
recommendations to deal with this dilemma are given in Box 3.
The divergent views of infection control committees and hospital
practice found in our survey reinforce our view that it is difficult or
impossible for individual hospitals to assess in isolation whether
reuse of single-use medical devices is safe and cost efficient. Our
recommendations to deal with this dilemma are given in Box 3.
We concur with the recent NHMRC draft report17 recommending that, if the Australian health ministers allow continuation of reuse, the institutions seeking to process devices for reuse should be licensed and comply with specific national standards and procedures, and that the cleaning/sterilising process should be cost effective.17
Cross-infection The frequency of cross-infections occurring from reuse of single-use medical devices is not known. There are no studies that address the "harmfulness" of reuse in a rigorous fashion (i.e., evidence-based medicine). No cases were reported in our study and there are none to our knowledge reported in the literature. If cross-infection does occur, it is likely to be infrequent. It may also go undetected because of the long incubation period and the asymptomatic nature of many blood-borne viral infections.
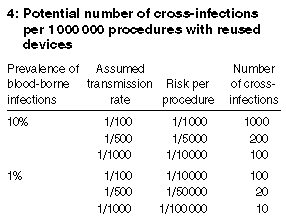
The number of cases of cross-infection occurring will depend on the prevalence of infection in the community, the risk of transmission and the number of procedures performed (Box 4). Unfortunately, we do not have information on all these factors. The risk is likely to be low and would be expected to be much less than that for a needlestick injury. The risk of transmitting HIV with a percutaneous injury when the source person is infected with HIV is about 1/300.22 Other viruses such as hepatitis B have much higher transmission rates,22 but, considering that reused single-use devices are cleaned and disinfected and that cross-infections have not been documented, the risk rate is likely to be 1/500 or less (and possibly close to zero). If one million procedures are carried out in Australia each year with reused single-use medical devices and we assume a 2% prevalence of blood-borne infections (our estimate for the prevalence of blood-borne infections in the Australian patient population), then 40 infections will be transmitted.
Costs and cost benefit The total extra costs involved in ceasing reuse are not known. One of the hospitals participating in the survey stopped reusing "single use only" medical devices which enter sterile sites. The estimated budget increases for consumables in that hospital's Gastroenterology Endoscopy Unit and Radiology Unit were $192 000 per year and $142 000 per year, respectively. Estimates have been made of additional costs of at least $100 million per year if reuse is to cease in Australia.20 Therefore, in Australia the maximum cost is likely to be $2.5 million per infection prevented. If the risk rate is much lower than 1/500 then the cost per infection prevented will be much higher.
Another factor to be taken into account is how many times a device is reused. If it is reused on 20 different patients, the cumulative risk of the device becoming contaminated with a blood-borne infection will be higher than in our estimates.
Banning the reuse of all "single-use" invasive medical devices will thus have major implications for health care costs, and it is unlikely that governments and third parties will contribute this extra funding. This may result in fewer diagnostic or therapeutic procedures being carried out, longer waiting lists for procedures, and the possibility of adverse outcomes for patients because procedures are not performed at the appropriate time. In addition to the direct dollar costs, these potential negative outcomes (associated with complete banning of reuse of single-use devices) need to be balanced against the infection risks and the risks of malfunction associated with reprocessing of these devices. The recent NHMRC report also stresses the need for extra resources if the Australian health ministers ban reuse of single-use devices.20
Acknowledgements
Register to be notified of new articles by email - - ©MJA1996
See also articles by Brook and Woollard
References
- Lacroix D, Lucas H, Stewart I. Anxiety, misinformation and
greed. Aust Nurs J 1994; 2: 17-20.
-
Campbell BA, Wells GA, Palmer WN, Martin DL. Reuse of disposable
medical devices in Canadian hospitals. Am J Infect Control
1987; 15: 196-200.
-
United States Food and Drug Administration. Compliance Policy
Guide. Reuse of medical disposable devices. 7124.6. Washington,
DC: FDA, 24 Sept 1987.
-
Australian Confederation of Operating Room Nurses policy
statement. Reuse of single use items. ACORN J 1992; Dec:
3.
-
Federation of Sterilising Research and Advisory Councils of
Australia (FSRACA) policy statement: reuse of single use items.
Melbourne: FSRACA, 14 Feb 1994.
-
Medical Industry Association of Australia (MMIA). Statement of
Industry Policy: reuse of single-use medical devices. Sydney: MMIA,
6 Aug 1991.
-
Adams A (Chief Medical Adviser, Commonwealth Department of Human
Services and Health). Communication: reuse of single-use devices.
Canberra: DHSH, 1 Aug 1994.
-
de Souza D. Reuse of medical devices [letter]. Med J Aust
1984; 141: 394.
-
9. Owen JW (Director-General, New South Wales Health). Reuse of
single use medical devices. Sydney: NSW Health, 22 Jul 1994. (Draft
Circular 83/62.)
-
Lange D (Chief Health Officer, Queensland Health Sterilising
Services). The reuse of single use medical devices. Brisbane:
Queensland Department of Health, 5 August 1993. (Medical Circular
No. 16/93.)
-
Lynch P (Acting Chief Medical Officer, Victorian Department of
Health and Community Services). Re-use of disposable single use
items. Melbourne: DHCS, 29 January 1993. (Circular No. 2/1993.)
-
Commonwealth of Australia. Therapeutic Goods Act. Canberra:
AGPS, 1989.
-
Hospital and health services yearbook and equipment
catalogue, 14th ed. Prahran, VIC: Peter Isaacson Publications,
1990.
-
Chant K, Lowe D, Rubin G, et al. Patient to patient transmission of
HIV in private surgical consulting rooms. Lancet 1993; 342:
1548-1549.
-
Collignon P. Patient to patient transmission of HIV.
Lancet 1994; 343: 415.
-
Chant K, Kociuba K, Munro R, et al. Investigation of possible
patient-to-patient transmission of hepatitis C in a hospital.
NSW Public Health Bull 1994; 5: 47-51.
-
Dunnigan A, Roberts C, McNamara M, et al. Success of reuse of
cardiac electrode catheters. Am J Cardiol 1987; 60: 807-810.
-
Anderson F, Heale, J Alison, Harper RW. Feasibility and cost
effectiveness of reuse of electrophysiology electrode catheters
[abstract]. The Cardiac Society of Australia and New Zealand Meeting
1994. Aust N Z J Med 1994; 24: 653.
-
Myburgh RE, Eldridge KL, Weerasooriya HR, Davis MJE. Reuse of
electrophysiologic and ablation catheters -- feasibility and costs
[abstract]. The Cardiac Society of Australia and New Zealand Meeting
1994. Aust N Z J Med 1994; 24: 655.
-
National Health and Medical Research Council. Report of the
NHMRC Expert Panel on the Re-use of Medical Devices Labelled as Single
Use. Draft report. Canberra: NHMRC, 26 Oct 1995.
-
Atkins, R. Examination of single use items by scanning electron
microscopy. J GENSA 1995; 5: 13-15.
-
Henderson DK. HIV-1 in the health care setting. In: Mandell GL,
Bennett JE, Dolan R, editors. Principles and practice of infectious
diseases, 4th ed. New York: Churchill Livingston, 1995: 2632-2656.
(Received 27 Nov 1995, accepted 21 Mar 1996)
Authors details
Woden Valley Hospital, Canberra, ACT.
Peter J Collignon, FRACP, FRCPA, Microbiologist and
Infectious Diseases Physician; and Head, Infectious Diseases Unit;
Elaine Graham, RN, CIC, Clinical Nurse Consultant,
Infection Control;
Dianne E Dreimanis, RN, RM, BHSc(Nursing), Acting Clinical
Nurse Consultant, Infection Control.
No reprints will be available. Correspondence: Dr P J Collignon,
Infectious Diseases Unit, Woden Valley Hospital, PO Box 11, Woden,
ACT 2606.
-
-
©MJA1996
See also articles by Brook and Woollard
< URL: http://www.mja.com.au/>
© 1996 Medical Journal of Australia.
(Received 27 Nov 1995, accepted 21 Mar 1996)
Authors details
Woden Valley Hospital, Canberra, ACT.Peter J Collignon, FRACP, FRCPA, Microbiologist and Infectious Diseases Physician; and Head, Infectious Diseases Unit; Elaine Graham, RN, CIC, Clinical Nurse Consultant, Infection Control;
Dianne E Dreimanis, RN, RM, BHSc(Nursing), Acting Clinical Nurse Consultant, Infection Control.
No reprints will be available. Correspondence: Dr P J Collignon, Infectious Diseases Unit, Woden Valley Hospital, PO Box 11, Woden, ACT 2606.
- - ©MJA1996
See also articles by Brook and Woollard < URL: http://www.mja.com.au/> © 1996 Medical Journal of Australia.
- Peter J Collignon
- Elaine Graham
- Dianne E Dreimanis




