Drug-resistant Streptococcus pneumoniae: the beginning of the end for many antibiotics?
Peter J Collignon and Jan M Bell, on behalf of the Australian Group on Antimicrobial Resistance (AGAR)*
Abstract - Introduction - Methods - Antibiotic sensitivity testing - Statistical analysis - Results - Discussion - Acknowledgements - References - Authors' details
- ©MJA1997
Abstract |
Objective: To determine the levels of antibiotic
resistance in Streptococcus pneumoniae in Australia. Design: Prospective, Australia-wide, laboratory-based survey. Setting: 27 hospital and private laboratories around Australia, from January 1994 to August 1995. Subjects: First 100 patients with clinically significant isolates of S. pneumoniae at each laboratory. Outcome measures: Resistance to penicillin (determined from penicillin minimum inhibitory concentration [MIC] measured by the Etest), erythromycin, trimethoprim-sulfamethoxazole, tetracycline, chloramphenicol, cefotaxime and ceftriaxone. Results: A total of 2396 isolates were tested (including 537 invasive isolates and 740 from children). Penicillin resistance was seen in 161 isolates (6.7%), including 17 with high level resistance. Penicillin resistance rates were significantly lower in invasive than in non-invasive strains (3.7% versus 7.6%; odds ratio [OR], 0.47; 95% confidence interval [CI], 0.28-0.77; P = 0.001). There was no significant difference in penicillin resistance rates between children ( < 15 years) and adults (7.3% versus 6.5%; OR, 1.14; 95% CI, 0.80-1.63; P = 0.47). Resistance rates were higher for most other antibiotics than for penicillin (chloramphenicol, 6%; erythromycin, 11%; tetracycline, 15%; and trimethoprim-sulfamethoxazole, 42%). No high level resistance was seen to third generation cephalosporins, but 17 of 109 penicillin-resistant isolates tested (16%) displayed intermediate resistance to cefotaxime. Rates of antibiotic resistance varied between States, with the lowest rates in Tasmania. Conclusions: Antibiotic resistance levels in S. pneumoniae are increasing in Australia and high level penicillin resistance is being encountered for the first time (including in invasive strains). This will lead to an increasing number of therapeutic dilemmas and possible therapeutic failures, especially important in meningitis. |
Introduction |
The pneumococcus (Streptococcus pneumoniae) continues to
be a common cause of serious and life-threatening infections,
including pneumonia, bacteraemia and meningitis. It is also a
frequent cause of respiratory tract infections, such as otitis media
and sinusitis.1-4 A major
advance was made in the treatment of these infections with the
introduction of penicillin 50 years ago. Until relatively recently,
pneumococci were considered so uniformly sensitive to penicillin
(with minimum inhibitory concentrations [MICs] < 0.02 mg/L) that
sensitivity tests were usually not performed.
It was from Australia in 1967 that the first clinically significant isolate of a penicillin-resistant pneumococcus was reported.5 However, penicillin resistance was not a major clinical problem in this country, although it caused major problems elsewhere, particularly in Papua New Guinea and South Africa.1-3 In the late 1970s and the 1980s, rates of resistance (including multiple resistance) increased in Western countries, particularly in Spain (with resistance levels of 50%).1,2,3 A recent United States study found 25% of invasive S. pneumoniae isolates were penicillin-resistant.6 Resistance rates are usually higher in children, and the distribution of resistance varies within countries and population groups.1,2,3,6 In an Australia-wide study of over 1800 isolates of S. pneumoniae in 1989, we found that only 1% were penicillin-resistant,7 a lower rate than in most other Western countries. However, some communities (especially Australian Aboriginals) have relatively high rates of resistance.8 Because of the worldwide increase in resistance to many antibiotics and the implications of penicillin resistance in S. pneumoniae for treatment of life-threatening conditions (particularly meningitis), we undertook a further study of resistance to penicillin and other commonly used antibiotics in clinically significant S. pneumoniae isolates from both the community and hospitals. |
Methods |
Twenty-seven hospital and private laboratories from around
Australia parti cipated. From January 1994, each laboratory tested
the first 100 consecutive clinically significant isolates. The rate
of collection varied from 5 to 20 months, with all laboratories
filling their quota in August 1995.
Patients' sex, age, specimen site and inpatient or outpatient status were recorded prospectively. Clinically significant isolates were defined as those isolated either from normally sterile sites (e.g., cerebrospinal fluid and blood [invasive isolates]) or from specimens that made contact with mucosal surfaces (e.g., sputum) if they were associated with an increased white cell count on gram staining and would normally have been reported as clinically significant. Throat or surveillance swabs were excluded, as were duplicates of clinically significant isolates. S. pneumoniae was identified by colonial morphology, a -haemolysis on blood agar plates, susceptibility to optochin and/or bile solubility. |
Antibiotic sensitivity testing |
Isolates were tested for susceptibility to penicillin,
erythromycin, trimethoprim-sulfamethoxazole, tetracycline and
chloramphenicol by the standardised routine method of each
laboratory. Methods included disc diffusion with either National
Committee for Clinical Laboratory Standards (NCCLS)9 (14 laboratories) or Calibrated
Dichotomous Sensitivity (CDS)10,11 (7 laboratories); agar
dilution12 with either
Isosensitest agar (Oxoid) (2 labora tories) or Mueller-Hinton agar
(3 lab oratories); and the ATB system (BioMerieux sa ,
Marcy-l'Etoile, France) (1 laboratory).
The MIC of penicillin was also determined for each isolate by the Etest on Mueller-Hinton agar supplemented with 5% blood;13,14 plates were incubated at 35¡C in 5% CO 2 for 20-24 hours. 14 The interpretive criteria of the NCCLS15 were used for susceptibility categorisation of Etest values (susceptible, MIC < 0.06 mg/L; intermediate resistance, MIC = 0.125-1 mg/L; and high level resistance, MIC > > 2 mg/L). For the study, antibiotic resistance was defined as decreased susceptibility (both intermediate and high level resistance),6,12 and multidrug resistance as decreased susceptibility to two or more of the antibiotic agents tested. Isolates from normally sterile sites and those that appeared resistant to penicillin or chloramphenicol by routine susceptibility testing or had penicillin MICs > > 0.047 mg/L were forwarded to Monash Medical Centre for further susceptibility testing: Etest strips were used to determine cefotaxime and ceftriaxone MICs. |
Statistical analysis | Fisher's two-tailed exact test was used to calculate P values. Calculations were performed with True Epistat software.16 |
Results |
A total of 2396 isolates from different patients were tested. The
average age of the patients was 41.6 years (range, < 1 day to 98
years); 32% were children ( < 15 years) and 60% were male.
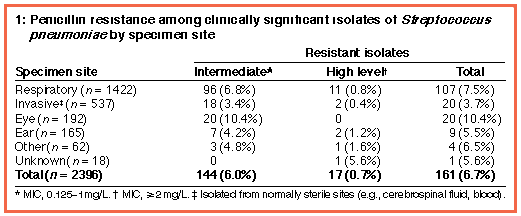
The percentage of penicillin-resistant isolates from each specimen site is shown in Box 1; the overall rate of penicillin resistance was 6.7%, with rates in individual laboratories ranging from 0 to 13%. High level resistance was seen in 17 isolates, including two from normally sterile sites.
The rate of penicillin resistance was significantly lower among invasive isolates than among non-invasive isolates (3.7% versus 7.6%; odds ratio [OR], 0.47; 95% confidence interval [CI], 0.28-0.77; P = 0.001) (Box 2). The rate of penicillin resistance was slightly higher among children ( < 15 years) than among adults, but the difference was not statistically significant (7.3% versus 6.5%; OR, 1.14; 95% CI, 0.8-1.63; P = 0.47).
Resistance to antibiotics other than penicillin was common (Boxes 2 and 3). Rates varied around Australia, with the lowest rates for nearly all antibiotics in Tasmania and the highest in the eastern States, particularly Queensland and New South Wales. The rate of penicillin resistance was highest in South Australia. Very high levels of resistance were seen for trimethoprim-sulfamethoxazole (29%-52%).
All five antibiotics were tested on 1895 isolates; 267 (14%) were
multi resistant, with 159 (8%) resistant to three or more antibiotics
and 31 (1.6%) to all five (Box 3). Of 124 penicillin-resistant
isolates tested, 72 (58%) were resistant to three or more non- Of 109 penicillin-resistant isolates tested with cefotaxime, 17 (16%) had intermediate resistance (MIC, 1 mg/L). These comprised 12 of 13 isolates with high-level penicillin resistance and 5 of 96 with intermediate penicillin resistance. Only three of the 109 isolates had intermediate resistance to ceftriaxone (all with high-level penicillin resistance). |
Discussion |
We found that the level of penicillin resistance among S.
pneumoniae isolates was six times higher than that found in 1989
in the only other large multicentre Australian study,7 but fortunately it was still lower
than in most other countries. Penicillin resistance rates
are very high in Third World countries, and in some areas of Western
Europe and the USA.1-3
However, the rate of rise in resistance in Australia appears very
similar to that seen in the early 1980s in countries such as Spain1-3 and in the early 1990s in the
United States;6 there, only
0.02% of isolates nationally were penicillin-resistant in the early
1980s and still only 1.3% in 1992,6
but a recent study found a rate of 25%, with much higher rates in
some subgroups (e.g., 40% in white children). Of equal concern was
that 3% of isolates had high level resistance to both penicillin and
third generation cephalosporins.6
Over the next few years, we are likely to see similar rates of
resistance developing in Australia.
The finding of high level penicillin resistance among S.
pneumoniae isolates in Australia is of particular concern; in
meningitis caused by organisms with any level of penicillin
resistance, penicillin treatment is likely to fail.1-3,17,18 Penicillin resistance has
consequences for other related drugs, as in S. pneumoniae it
is not due to Of even greater concern are the implications of the rapid rise in resistance to third generation cephalosporins noted in the United States. Primary resistance to these drugs is less frequent than to penicillin, but requires less genetic change.1,19 In some areas up to 27% of penicillin-resistant pneumococci have high level resistance to cefotaxime.1 This leads to therapeutic failure of these agents, yet they are the main treatment for the increasingly common intermediate penicillin-resistant strains. No high level cefotaxime-resistant strains were seen in our study or have been reported in Australia, to our knowledge. However, given the worldwide spread of resistant pneumococci in the recent past, they will inevitably be seen soon in Australia and leave us with major therapeutic dilemmas in the treatment of meningitis. In life-threatening situations other than meningitis (e.g., bacteraemia), high dose intravenous penicillin appears sufficient to eradicate organisms with intermediate resistance, as drug levels achievable in serum are still much higher than the MIC.1,2,3 There is, however, controversy, and many recommend use of either cefotaxime or ceftriaxone.1,3 For organisms with high level resistance, the most appropriate agent is unclear. However, we would favour vancomycin.
In non-life-threatening infections with penicillin-resistant
pneumococci, the most appropriate antibiotics are less clear. In
otitis media, amoxycillin still appears the best choice,20,21 as drug levels achieved in the
middle ear can still exceed the MICs of strains with intermediate
resistance (although higher doses may be needed). Other oral agents
available in Australia for use in children (cefaclor, trimethoprim,
erythromycin and cefpodoxime) do not reach adequate levels to
eradicate resistant isolates.20,21 Third generation
cephalosporins, such as ceftriaxone, are active, but their
parenteral route is likely to preclude their use. Combining
clavulanic acid with amoxycillin is no advantage, as the resistance
is not due to The reasons for the increasing resistance in S. pneumoniae worldwide are not completely understood, although antibiotic pressure appears to be a major factor.1 A few resistant clones were shown to have spread from one continent to others (e.g., from Spain to the United States and Iceland) and then through the local population, undergoing minor genetic changes in the process.1,3,19 The pneumococcus can acquire DNA molecules from other bacteria that probably include viridans group streptococci (e.g., Streptococcus mitis), which form part of the normal flora of the nasopharynx.1,19 While it would probably be impossible to eradicate carriage of these resistant organisms from the population, it may be possible to reduce the rate of increase in resistance by minimising the prescription of unnecessary antibiotics. Other strategies, such as vaccination, may be necessary. Unfortunately, the currently available vaccine is a polysaccharide and therefore a poor immunogen, especially in young children. Studies are under way to assess a conjugated pneumococcal vaccine (i.e., a carbohydrate with protein carrier), but vaccine development is difficult as there are over 80 serotypes of pneumococci (compared with only one commonly invasive serotype of Haemophilus influenzae -- type b). However, at present most of the resistant organisms belong to relatively few serotypes.1,2,3 A vaccine containing most of these might not only decrease life-threatening disease, but might also decrease carriage of the organisms, as was found for the H. influenzae type b (Hib) vaccine.1 However, as the pneumococcus can acquire DNA from other organisms,1,19 the number of resistant serotypes is likely to increase. Our study was one of the largest in the world where all organisms were clinically significant and all were assessed for MIC for penicillin. It is valuable not only for showing the rate of resistance (both intermediate and high level), but also for providing a baseline to assess future changes in resistance and to differentiate subtle shifts in resistance in the whole population of pneumococci from the introduction of resistant clones. In the past, determining MICs was time-consuming, laborious and not routine. The recent development of the Etest (which consists of a strip of paper impregnated with increasing concentrations of antibiotic from one end to the other) has simplified the procedure. This technological advance, along with the willingness of so many laboratories around Australia to participate in the project, has allowed us to obtain information essential for guiding us in making appropriate antibiotic choices and designing empiric therapy for these emerging threats. |
Acknowledgements | We wish to thank the many doctors, scientists and technicians at the participating laboratories who donated their time and resources to carry out this project. The penicillin Etest strips were supplied by Australian Laboratory Services Pty Ltd at cost price. Eli Lilly provided funding for many of the participants to meet at the twice-yearly AGAR meeting. Cefotaxime and ceftriaxone Etest strips were donated by AB Biodisk (Sweden). |
References |
(Accepted 30 Nov 1995) |
Authors' details
Infectious Diseases Unit, Woden Valley Hospital, Canberra, ACT.Peter J Collignon, FRACP, FRCPA, Head of Unit, Microbiologist and Infectious Diseases Physician.
Department of Microbiology and Infectious Diseases, Monash Medical Centre, Melbourne, VIC.
Jan M Bell, BSc(Hons), BA, Scientist.
* Australian Group on Antimicrobial Resistance (AGAR).
For this study AGAR consisted of the microbiology laboratories
at:
ACT: Woden Valley Hospital (Peter Collignon,
Linda Halliday).
NSW: Concord Hospital (Joan Yap, Tom Gottlieb, Glenn
Funnell); Illawarra Regional Hospital (Keith Wise, Rodney Jones);
Liverpool Hospital (Denise Daley, Rosemary Munro); Prince of Wales
Hospital (Jeanette Pham, Barrie Gatus, Sydney Bell); Royal North
Shore Hospital (Clarence Fernandes); Royal Prince Alfred Hospital
(Richard Benn, Barbara Yan, Alison Vickery).
QLD: Mater Misericordiae Hospital, Brisbane (Martyn Tilse,
Janet Montgomery); Princess Alexandra Hospital (Graeme Nimmo,
Jacqueline Schooneveldt); Royal Brisbane Hospital (Narelle
George, Joan Faoagali); Sullivan, Nicolaides and Partners (Jenny
Robson, Sylvia van der Valk); Toowoomba Base Hospital (David
Farrell).
SA: Flinders Medical Centre (Hendrik Pruul); Institute of
Medical and Veterinary Science (Irene Lim, Richard Lumb); Queen
Elizabeth Hospital (Peter Lawson, David Grove).
TAS: Diagnostic Pathology (which includes Hobart Pathology
and Launceston Pathology) (Barbara Henderson, Danny McColl, Gary
Fenton); Launceston General Hospital (Erika Cox, Veronica Lyons);
Royal Hobart Hospital (Keith Ott, Rob Peterson).
VIC: Alfred Hospital (John Spicer, J Clare Franklin);
Dorevitch Pathology (Liz Snashall); Heidelberg Repatriation
Hospital (Barrie Mayall, Angie Chan, Vicki Moritz); Melbourne
Pathology (Christine Hargreaves); Monash Medical Centre (Dianne
Olden, Jan Bell, John Turnidge); Royal Children's Hospital and
Microbiological Diagnostic Unit (Geoff Hogg, Marion Easton, Janet
Strachan).
WA: Fremantle Hospital (David McGechie, Neil Stingemore,
Graham Francis); Royal Perth Hospital (Keryn Christiansen, Claire
Khinsoe, Geoff Coombs).
Reprints: Dr P J Collignon, Infectious Diseases Unit, Woden Valley Hospital, PO Box 11, Woden, ACT 2606.
©MJA 1997
<URL: http://www.mja.com.au/> © 1997 Medical Journal of Australia.
Received 8 February 2026, accepted 8 February 2026
- Peter J Collignon
- Jan M Bell
- the AGAR







 -lactam
agents; 40% were resistant to chloramphenicol; 52% to erythromycin;
64% to tetracycline; and 78% to trimethoprim-sulfamethoxazole. Of
the 1771 pencillin-susceptible isolates, 101 (6%) were resistant to
three or more non-
-lactam
agents; 40% were resistant to chloramphenicol; 52% to erythromycin;
64% to tetracycline; and 78% to trimethoprim-sulfamethoxazole. Of
the 1771 pencillin-susceptible isolates, 101 (6%) were resistant to
three or more non-