Furthermore, clinical trial registries are well placed to provide representative information about trial sponsorship and funding sources. A United Kingdom study1 found that support for randomised trials from non-industry sources in the UK had fallen, suggesting that an increasing proportion of trials were funded by industry, raising concerns about the desirability of this trend and whether policy changes should address it. In the United States, funding from industry sources (pharmaceutical, biotechnology and medical device firms) increased each year from 2003 to 2008.2 The concern is that studies suggest industry-funded trials tend to draw pro-industry conclusions.3
The largest trial registry, ClinicalTrials.gov (CT.gov),4 was established in the US in 2000.5 There is also a large European registry,6 and smaller local registries are being established in several countries. The Australian New Zealand Clinical Trials Registry (ANZCTR),7 a World Health Organization primary registry, was created in 2005, and 3668 trials were registered to the end of 2009.
Data were extracted from the ANZCTR and CT.gov for trials that fulfilled three criteria: cancer, interventional trials, and recruiting in Australia at 31 March 2009. Original data were retained wherever possible; however, some data items were collected differently by each registry and were recoded for consistency (details of recoding are provided at http://sydney.edu.au/science/psychology/cemped/research.shtml#patientcommunication).
The cancer types used in our report have been standardised to those used by the Australian Institute of Health and Welfare and refer to the primary site of the cancer.8
We used the WHO definition of primary sponsor: “The individual, organization, group or other legal entity which takes responsibility for initiating, managing and/or financing a study.”9 Primary sponsors’ responsibilities include initiating and managing a study, appropriate conduct and reporting, and obtaining ethics approval to commence a study. We categorised primary sponsor as industry (pharmaceutical and device companies) or non-industry (universities, collaborative groups, charities and government organisations). The primary sponsor of a trial may or may not be its main funder. The main funder is defined as the “major source(s) of monetary or infrastructure support for the trial”,9 such as a funding agency, foundation, company, hospital or university.
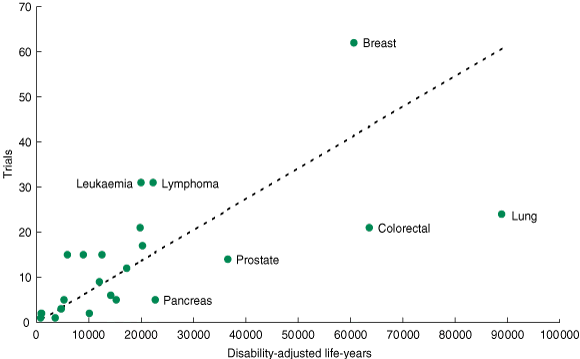
We described trials in terms of: cancer type, intervention tested, sponsorship and funding source, phase and design (randomised versus non-randomised), characteristics of enrolled subjects, trial registry (the ANZCTR or CT.gov) and location of recruitment (Australia only or Australia and overseas). For each cancer, we obtained data on disability-adjusted life-years (DALYs) for 2003.10 DALYs are the sum of the years of life lost due to premature mortality in the population and the equivalent “healthy” years lost due to disability for incident cases. We tested whether the number of clinical trials for each cancer was proportional to the number of DALYs for that cancer using the χ2 goodness-of-fit test. The following cancer types with small numbers of trials were grouped together for this analysis: prostate and testis; head and neck, eye and thyroid; pancreas, liver and gallbladder; and cervix and uterus. The cancer types of all/multiple, other, other haematological, mesothelioma and unknown primary sites were not included in this analysis as DALY information was not available.
SAS, version 9.2 (SAS Institute Inc, Cary, NC, USA) was used for statistical data analysis.
Most trials were in breast cancer, followed by lymphoma and leukaemia, lung cancer, colorectal cancer, brain cancer and melanoma (Box 1). Breast cancer trials anticipated recruiting the largest number of patients: 74 247 patients (38%) out of an expected total sample size of 195 982 for all trials combined.
Many of the cancers with higher burdens of disease (measured by DALYs) were underrepresented (Box 1 and Box 2). This was most notable for lung, colorectal, prostate and pancreatic cancers. Conversely, leukaemia and lymphoma were overrepresented relative to their DALYs. There was a significant difference between the observed number of trials in each cancer type and the number of trials expected based on DALYs (χ2 = 154.7; P = 0.001). We found a similar pattern for the anticipated sample size relative to DALYs (http://sydney.edu.au/science/psychology/cemped/research.shtml#patientcommunication).
Industry sources were the primary sponsor in 43% of trials (Box 3). There was strong evidence of a difference in the primary sponsor between the cancer types (χ2 = 26.7; P = 0.005). Of 368 trials, there were 337 (92%) with the same funding source and primary sponsor. Industry funded 98% of industry-sponsored trials and 13% of non-industry-sponsored trials.
Most drug trials were sponsored or funded by industry (56% and 64%, respectively). For all other interventions analysed, non-industry was the main source of sponsorship (Box 4) and funding.
Bivariate analysis showed a relationship between primary sponsorship by industry and cancer group (P = 0.001), intervention type (P < 0.001) and cancer extent (P < 0.001) (Box 5).
In the multivariate analysis, the significant factors were intervention type (P < 0.001), cancer extent (P = 0.001) and allocation to intervention (P = 0.030) (Box 5). Trials of systemic interventions were more likely than local therapies to be sponsored by industry (OR, 16.71; 95% CI, 4.70–59.43). Trials that included patients with advanced disease were more likely to be sponsored by industry than trials that included patients with early disease (OR, 3.76; 95% CI, 1.78–7.94), and randomised controlled trials had higher odds than non-randomised trials of being sponsored by industry (OR, 1.78; 95% CI, 1.06–3.00).
Drug treatments were the most commonly tested intervention (69%), followed by biological treatments, behavioural interventions, radiation, chemoradiation, surgery and diagnosis (Box 6). The least common interventions tested included prevention, early detection and lifestyle (1% each).
Most trials investigating drugs, biological agents and radiation were registered with CT.gov and were open for recruitment internationally. In contrast, most trials investigating behaviour, lifestyle, diagnosis, early detection and prevention were registered with the ANZCTR and recruiting only in Australia (Box 6).
Results describing trials according to cancer extent, age restrictions and phase of trials can be found at http://sydney.edu.au/science/psychology/cemped/research.shtml#patientcommunication.
We conducted a search of the WHO International Clinical Trial Registry Platform (ICTRP) for cancer trials recruiting at 31 March 2009.11 We found a similar proportion of trials were recruiting for each cancer type as in our analysis (see http://sydney.edu.au/science/psychology/cemped/research.shtml#patientcommunication), suggesting that our data are representative of cancer clinical trials internationally.
In contrast, there were more trials relative to burden of disease for breast, brain, kidney and haematological malignancies and sarcoma. The highest proportion of registered, open-to-recruitment, interventional trials were in breast cancer. Breast cancer was also notable because it anticipated recruiting a very large number of patients relative to its burden of disease. Our findings are consistent with reports comparing the number of trials with cancer incidence and mortality data.12
Trials in breast cancer differed in important ways. For all cancer types except breast cancer, most trials included people with advanced disease, whereas most breast cancer trials included women with early-stage disease. A large proportion of breast cancer trials were investigating non-drug interventions — all prevention trials, two-thirds of lifestyle trials and almost 30% of behavioural trials. Relatively, a higher proportion of breast cancer trials were non-industry sponsored and funded. These features might be a consequence of consumer influence on the research agenda. For example, the US National Breast Cancer Coalition, set up in 1991, has been instrumental in increasing federal funding for breast cancer research in the US.13
There was strong evidence that intervention type, cancer extent and allocation to intervention were related to sponsorship by industry after allowing for cancer type. It was surprising that cancer type was not significantly associated with industry sponsorship; type of treatment rather than type of cancer was more strongly associated with industry sponsorship.
The number of trials and their associated sample sizes are easily derived measures of the resources allocated to a particular cancer. A more comprehensive enumeration of the resources involved in each trial may provide different results.
Our study might not include all interventional trials open for recruitment in Australia. Unlike in the US (where trial registration of drugs and devices is legally required, except for Phase 1 trials14), it is not a legal requirement to register clinical trials in Australia, although policies to increase registration rates have been implemented. In 2007, the revised National statement on ethical conduct in human research, which governs the conduct of human research ethics committees in Australia, recommended that clinical trials be registered before enrolment of the first patient.15 As in other countries, we anticipate the majority of large, Phase 3 trials recruiting in Australia would be registered because proof of registration is increasingly required by institutional ethics committees. In addition, the Australian code for the responsible conduct of research states that “Researchers must register clinical trials with a recognised register to promote access to information about all clinical trials”.16 These policies have added to international initiatives, including the International Committee of Medical Journal Editors requirement for prospective trial registration17 and the Declaration of Helsinki, which includes trial registration as a core ethical principle.18 Unfortunately, the number of unregistered trials currently in progress in Australia remains unknown and is not estimable. Despite this limitation, we have observed some very strong associations in our data, and there would have to be a large number of unregistered trials with very different features from those described here to substantially alter the pattern of our results.
A search of the ICTRP found that 98% of registered trials open to recruitment in Australia were registered with the ANZCTR and CT.gov.11 Given that only 2% were registered with the other trial registries, it is unlikely that the exclusion of these trials would have affected our results.
Our findings demonstrate the potential of registry data to identify gaps in current trial activity and to guide future trials research. Recent studies19,20 used CT.gov to describe cancer vaccine trials and ongoing clinical trials in non-small cell lung cancer but did not provide a national picture or a comprehensive analysis for cancer. Our description of the national clinical trial landscape in cancer is unique. This approach may provide a useful addition for setting future research priorities and provides a baseline against which to measure future trends.
1 Cancer types ranked by number of clinical trials, burden of disease (as DALYs) and total target sample size
4 Primary sponsorship of intervention types used in cancer clinical trials
* Acupuncture, nasopharyngeal humidification and enteral nutrition. |
|||||||||||||||
Provenance: Not commissioned, externally peer reviewed.
Received 26 August 2010, accepted 24 January 2011
- Rachel F Dear1
- Alexandra L Barratt2
- Kevin McGeechan3
- Lisa Askie4
- John Simes5
- Martin H N Tattersall6
- University of Sydney, Sydney, NSW.
This work was supported by the National Health and Medical Research Council (grant reference number 512380). We thank Sheena Arora for her assistance in recoding and merging data and Thuyen Vu of the ANZCTR for extracting data from the ANZCTR and CT.gov.
Lisa Askie and John Simes are Manager and Director, respectively, of the ANZCTR.
- 1. Chalmers IC, Rounding C, Lock K. Descriptive survey of non-commercial randomised controlled trials in the United Kingdom, 1980–2002. BMJ 2003; 327: 1017-1021.
- 2. Dorsey ER, de Roulet J, Thompson JP, et al. Funding of US biomedical research, 2003–2008. JAMA 2010; 303: 137-143.
- 3. Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA 2003; 290: 921-928.
- 4. US National Institutes of Health. ClinicalTrials.gov. http://www.clinicaltrials.gov (accessed May 2010).
- 5. Zarin DA, Ide NC, Tse T, et al. Issues in the registration of clinical trials. JAMA 2007; 297: 2112-2120.
- 6. Current Controlled Trials. International Standard Randomised Controlled Trial Number Register. http://www.isrctn.org (accessed Jun 2010).
- 7. National Health and Medical Research Council; New Zealand Health Research Council. Australian New Zealand Clinical Trials Registry. http://www.anzctr.org.au/default.aspx (accessed Jun 2010).
- 8. Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) books. http://www.aihw.gov.au/cancer/data/acim_books/index.cfm (accessed Jun 2010).
- 9. World Health Organization. The World Health Organization International Clinical Trials Registry Platform. WHO trial registration data set. http://www.who.int/ictrp/network/trds/en/index.html (accessed May 2010).
- 10. Begg S, Vos T, Barker B, et al. The burden of disease and injury in Australia 2003. Canberra: Australian Institute of Health and Welfare, 2007. (AIHW Cat. No. PHE 82.)
- 11. World Health Organization. International Clinical Trials Registry Platform search portal. http://apps.who.int/trialsearch (accessed May 2010).
- 12. Wellberry H, Catanzariti A, Edwards C, Bishop J. Cancer clinical trials in NSW, 2004–2006. Sydney: Cancer Institute NSW, 2008.
- 13. Liberati A. Consumer participation in research and health care. BMJ 1997; 315: 499.
- 14. US Congress. Food and Drug Administration Amendments Act 2007. Public Law 110-85. Section 801. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=110_cong_public_laws&docid=f:publ085.110.pdf (accessed Mar 2011).
- 15. National Health and Medical Research Council; Australian Research Council; Australian Vice-Chancellors’ Committee. National statement on ethical conduct in human research. Canberra: NHMRC, 2007. http://www.nhmrc.gov.au/publications/synopses/e72syn.htm (accessed Mar 2011).
- 16. National Health and Medical Research Council; Australian Research Council; Universities Australia. Australian code for the responsible conduct of research. Canberra: Australian Government, 2007. http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/r39.pdf (accessed Jan 2011.)
- 17. DeAngelis CD, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004; 292: 1363-1364.
- 18. World Medical Association. WMA Declaration of Helsinki. Ethical principles for medical research involving human subjects. Amended Oct 2008. http://www.wma.net/en/30publications/10policies/b3/17c.pdf (accessed Jan 2011).
- 19. Cao X, Maloney K, Brusic V. Data mining of cancer vaccine trials: a bird’s-eye view. Immunome Res 2008; 4: 7.
- 20. Subramanian J, Madadi AR, Dandona M, et al. Review of ongoing clinical trials in non-small cell lung cancer: a status report for 2009 from the ClinicalTrials.gov website. J Thorac Oncol 2010; 5: 1116-1119.





Abstract
Objective: To quantify and describe current cancer clinical trial activity in Australia and help guide future trials research using trial registries.
Design and setting: Data from cancer trials recruiting in Australia at 31 March 2009 were extracted from the Australian New Zealand Clinical Trials Registry and ClinicalTrials.gov. A regression model was used to identify factors associated with industry sponsorship.
Main outcome measures: The proportion of cancer trials compared with estimated burden of disease for each cancer.
Results: There were 368 interventional cancer trials open to recruitment. The most-researched cancer was breast cancer, accounting for 17% of trials. Only 7% of trials were in lung cancer, yet lung cancer is responsible for the greatest burden of disease. Industry was the primary sponsor in 43% of trials. Drug treatments were tested in most trials (69%). Trials were more likely to be industry sponsored if they tested systemic rather than local treatments (OR, 16.71; 95% CI, 4.70–59.43), included patients with advanced rather than early disease (OR, 3.76; 95% CI, 1.78–7.94) and used random rather than non-random allocation (OR, 1.78; 95% CI, 1.06–3.00).
Conclusion: There is variation in the number of trials according to cancer site, with some cancers being underrepresented relative to their burden of disease. Industry sponsorship is more likely for trials that investigate systemic therapy, recruit patients with advanced disease and are randomised.