A veterinarian became infected with Hendra virus (HeV) after managing a terminally ill horse and performing a limited autopsy with inadequate precautions. Although she was initially only mildly ill, serological tests suggested latent HeV infection. Nevertheless, she remains well 2 years after her initial illness. Recently emerged zoonotic viruses, such as HeV, necessitate appropriate working procedures and personal protective equipment in veterinary practice.
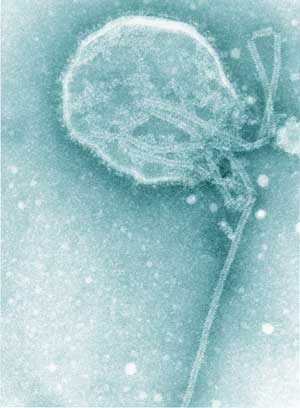
Hendra virus (HeV) and Nipah virus together comprise the genus Henipavirus within the family Paramyxoviridae (Box 1).1 HeV, formerly called equine morbillivirus, was first described after an outbreak of severe respiratory disease in horses, leading to the deaths of 14 horses and a horse trainer in Brisbane in September 1994.2,3 The trainer had had very close manual contact with frothy nasal and oral secretions, some of which were blood-tinged, from several of the very ill horses, as did a stable-hand; he developed an influenza-like illness but made a full recovery. The horses and both people were infected with HeV.2,3
An earlier outbreak of HeV disease was not recognised until the death, in 1995, of a farmer in Mackay. He had assisted his veterinarian wife with the autopsies of two horses that died suddenly from unknown cause in August 1994. He was hospitalised about 2 weeks later with an aseptic meningitis, from which he apparently made a full recovery.3,4 However, about a year later he became acutely unwell again, and died from a severe encephalitis caused by HeV.3,4 The two horses were (retrospectively) shown to have also been infected with HeV.5
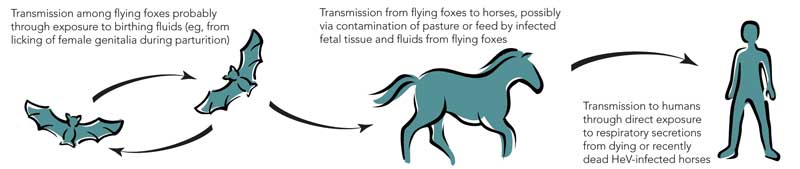
After these two outbreaks, extensive investigations identified fruit bats (Pteropus spp.), commonly known as flying foxes, as the likely natural reservoir of HeV; the infection is probably subclinical in most infected flying foxes.6,7 Although it is not certain how HeV could be transmitted from flying foxes to horses, the isolation of the virus from uterine fluid and aborted fetal tissue of flying foxes suggests that horses could ingest the virus on feed or pasture recently contaminated by birth products (Box 2).6,7 The available evidence indicates that transmission of the virus from horses to people, albeit rare, occurs through physical contact with nasal and oral secretions emanating from very ill, dying or dead horses.8
The third recognised outbreak of HeV disease occurred in January 1999, when a horse in a northern suburb of Cairns died from pneumonia.9 No human infection associated with this equine HeV case was detected.
Laboratory studies: HeV RNA was not detected by a reverse transcriptase polymerase chain reaction (RT-PCR) (TaqMan) assay10 on the initial serum sample. Similarly, HeV IgM and IgG antibodies were not detected by either immunofluorescence assay (IFA) or enzyme-linked immunoassay (EIA).11 However, the subsequent samples demonstrated clear HeV IgM and IgG seroconversions by both IFA and EIA. A serum sample taken nearly a year later was initially reported as having a very high antibody titre on IgG IFA (>1024), but on repeat testing this was revised to a level of 512 (Box 3). Subsequent antibody levels have fallen by one dilution. A plaque reduction neutralisation test11 showed that the serum collected 14 days after the onset of illness neutralised HeV at a dilution of 1 : 5.
Although no samples were collected from the horse, with hindsight it clearly had an illness consistent with previous clinical reports of HeV disease in horses. The disease is usually fulminant in nature, with fever, tachycardia, respiratory distress and a frothy nasal discharge being the typical reported features in horses.8,12 Facial oedema, physical distress and unease (suggestive of colic), and the close proximity of flying foxes add further support to the clinical suggestion of HeV disease in horses.12 The most obvious gross pathology is marked fluid congestion in the lungs, with a thick, foamy haemorrhagic exudate in the airways.8
Although there was no obvious flying fox colony nearby, large numbers of flying foxes are usually obvious in the evening sky in the latter part of the year in and around Cairns. The timing of the horse’s illness is not only the flying foxes’ birthing season,13 but also the season for many domestic and rainforest fruits in Far North Queensland; flying foxes travel considerable distances on nocturnal forays from their colonies in search of these foods. The owner of the horse reported frequently seeing flying foxes in the vicinity of the property.
This veterinarian is the fourth person known to have been infected with HeV. All four had direct exposure to secretions and tissues from very ill, dying or dead horses; two were directly involved in autopsies of these horses. Two of the four died, whereas the other two had relatively mild illnesses.2-4
The veterinarian has remained clinically well for 2 years since her initial illness. A rise in the antibody titre 1 year after the initial illness was of concern in view of the observed late neurological relapse (13 months after acute illness), associated with increasing antibody levels, in one of the other cases of HeV infection.4 The closely related Nipah virus has also been associated with late neurological relapse in 7.5% of cases, occurring up to 22 months after initial infection.14 In one case, late-onset Nipah virus encephalitis coincided with rising antibody titres.15
After this HeV incident, the Queensland Department of Primary Industries and Fisheries published revised guidelines for veterinarians handling horses suspected of being infected with HeV.12 These guidelines provide clinical case definitions and the recommended response measures, including the personal protective equipment that should be used when managing a suspected case, and the necessary reporting procedures.12 We suggest that these guidelines should be widely disseminated throughout the Australian veterinary community.
- 1. Wang L, Harcourt BH, Yu M, et al. Molecular biology of Hendra and Nipah viruses. Microbes Infect 2001; 3: 279-287.
- 2. Selvey LA, Wells RM, McCormack JG, et al. Infection of humans and horses by a newly described morbillivirus. Med J Aust 1995; 162: 642-645.
- 3. Paterson DL, Murray PK, McCormack JG. Zoonotic disease in Australia caused by a novel member of the Paramyxoviridae. Clin Infect Dis 1998; 27: 112-118.
- 4. O’Sullivan JD, Allworth AM, Paterson DL, et al. Fatal encephalitis due to a novel paramyxovirus transmitted from horses. Lancet 1997; 349: 93-95.
- 5. Hooper PT, Gould AR, Russell GM, et al. Retrospective diagnosis of a second outbreak of equine morbillivirus disease. Aust Vet J 1996; 74: 244-245.
- 6. Field H, Young P, Yob JM, et al. The natural history of Hendra and Nipah viruses. Microbes Infect 2001; 3: 307-314.
- 7. Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol 2000; 81: 1927-1932.
- 8. Westbury HA. Hendra virus disease in horses. Rev Sci Tech 2000; 19: 151-159.
- 9. Field HE, Barratt PC, Hughes RJ, et al. A fatal case of Hendra virus infection in a horse in north Queensland: clinical and epidemiological features. Aust Vet J 2000; 78: 279-280.
- 10. Smith IL, Halpin K, Warrilow D, Smith GA. Development of a fluorogenic RT-PCR (TaqMan) for the detection of Hendra virus. J Virol Methods 2001; 98: 33-40.
- 11. Daniels P, Ksiazek T, Eaton BT. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect 2001; 3: 289-295.
- 12. Queensland Department of Primary Industries and Fisheries. Handling suspect Hendra virus cases in all equines: guidelines for veterinarians. June 2005. http://www2.dpi.qld.gov.au/health/16503.html (accessed Jun 2006).
- 13. Hall L, Schulz M, Richards G. Bats. In: Ryan M, Burwell B, editors. Wildlife of tropical north Queensland. Brisbane: Queensland Museum, 2000: 313-327.
- 14. Tan CT, Goh KJ, Wong KT, et al. Relapsed and late-onset Nipah encephalitis. Ann Neurol 2002; 51: 703-708.
- 15. Wong SC, Ooi MH, Wong MN, et al. Late presentation of Nipah virus encephalitis and kinetics of the humoral immune response. J Neurol Neurosurg Psychiatry 2001; 71: 552-554.





We wish to thank the veterinarian, her colleague who first notified the incident, and the owners of the horse. We also wish to thank the medical practitioner concerned.
None identified.