Mycobacterium ulcerans is an environmental bacterium causing skin ulcers. An endemic area of M. ulcerans disease in temperate south-east Victoria, where the disease is known as "Bairnsdale ulcer", has been extensively studied, and continues to involve new geographic areas.1 A less well known endemic area exists in tropical far north Queensland between Mossman and the Daintree River (Douglas Shire) (Box 1), where it is called "Daintree ulcer".2
Our patient presented with diffuse limb swelling of acute onset without ulceration and associated with systemic symptoms, which is unusual for M. ulcerans disease in Australia; only two previous cases from far north Queensland have presented in this way.2 Most patients in Australia initially note a papular lesion, which subsequently ulcerates after weeks to months without associated systemic symptoms.3
The classic ulcer is painless, with a sharp, undermined edge and surrounding induration, indicating the extent of necrotic subcutaneous tissue. Histopathological examination of excised tissue from patients with M. ulcerans disease shows large numbers of extracellular mycobacteria, a poorly developed immune response and widespread necrosis of subcutaneous tissue,4 which may be mediated by a recently described lipid toxin (mycolactone) produced by M. ulcerans.5
The accepted treatment of M. ulcerans disease remains complete surgical excision of all necrotic tissue, often extending well beyond the visible margins of the ulcer, with primary closure of small lesions and immediate or delayed skin grafting to larger lesions. Antimycobacterial therapy appears ineffective,2 despite in-vitro sensitivity of the organisms to several antimycobacterial agents, possibly because of clustering of the organisms in necrotic fat, where penetration of antimicrobials is likely to be poor. We have used adjunctive therapy in addition to surgical excision in patients with locally extensive or disseminated disease, and/or incomplete surgical excision. In these situations, relapse and residual functional impairment are more common. The combination of rifampicin, ethambutol and clarithromycin, although not established in the treatment of M. ulcerans infection, has shown good activity against other non-tuberculous mycobacteria, and M. ulcerans is sensitive in vivo to rifampicin6 and in vitro to clarithromycin.7
Early diagnosis can reduce the extent of surgical excision required, and therefore the residual scarring and deformity, and may also minimise the risk of relapse. Increased awareness of the disease in endemic areas is important in early diagnosis. A diagnostic polymerase chain reaction (PCR) test,8 developed at the Royal Children's Hospital (Melbourne), has 96% sensitivity and 100% specificity for M. ulcerans and is available from the Victorian Infectious Diseases Reference Laboratory, Melbourne. It can be performed within one day from a dry swab of the ulcer, and allows clinicians to make a rapid decision about whether or not surgical excision is required. Laboratory culture confirmation remains important and provides isolates for further analysis, but it may take more than six weeks. Microscopy for acid-fast bacilli from ulcer swabs, although sensitive, is non-specific — it cannot distinguish M. ulcerans from other non-tuberculous mycobacteria. The high specificity of the diagnostic PCR is important, as, in contrast to M. ulcerans infection, other non-tuberculous mycobacteria usually respond to antimycobacterial therapy.
Clinical record
Examination showed an erythematous, tender swelling of his whole left forearm, with pitting oedema and indistinct margins from 10 cm above the elbow to 5 cm above the wrist (Box 2). M. ulcerans infection was considered in the differential diagnosis because of an awareness of the disease among healthcare workers in this area. Microscopic examination of Ziehl–Neelsen-stained skin biopsies revealed acid-fast bacilli, and a swab taken from the base of a punch biopsy site was positive for M. ulcerans by polymerase chain reaction (PCR), confirming the diagnosis of oedematous M. ulcerans disease. M. ulcerans was subsequently cultured from the skin biopsy.
1 : Mycobacterium ulcerans-endemic area
The location of the M. ulcerans-endemic area, Douglas Shire, far north Queensland, Australia.
2 : Mycobacterium ulcerans disease
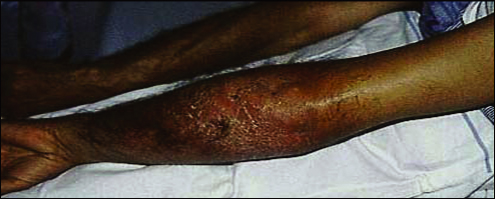
The patient's left arm before operation, showing extensive oedema and erythema.
Lessons from practice
Increased awareness of Mycobacterium ulcerans infection in the endemic areas (south-east Victoria and far north Queensland) is important in early diagnosis.
The disease may present with an acute onset and oedema, without ulceration.
Early diagnosis can reduce the extent of surgical excision and minimise the risk of relapse.
A diagnostic polymerase chain reaction (PCR) test with 96% sensitivity and 100% specificity for M. ulcerans is available from the Victorian Infectious Diseases Reference Laboratory (Melbourne).
- Grant A Jenkin1
- May Smith2
- Mark Fairley3
- Paul D R Johnson4
- 1 Department of Microbiology, Monash University, Clayton, VIC.
- 2 Mossman District Hospital, Mossman, QLD.
- 3 Roma Hospital, Roma, QLD.
- 4 Department of Infectious Diseases, Austin and Repatriation Medical Centre, Heidelberg, VIC.
- 1. Johnston PDR, Veitch MGK, Leslie DE, et al. The emergence of Mycobacterium ulcerans infection near Melbourne. Med J Aust 1996; 164: 76-78.
- 2. Smith M. Studies on Mycobacterium ulcerans infection in the Douglas Shire of Far North Queensland, Australia [MSc thesis]. Townsville: James Cook University of North Queensland, 1996.
- 3. Hayman J. Clinical features of Mycobacterium ulcerans infection. Australas J Dermatol 1985; 26: 67-73.
- 4. Hayman J, McQueen A. The pathology of Mycobacterium ulcerans infection. Pathology 1985; 17: 594-600.
- 5. George KM, Chatterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 1999; 283: 854-857.
- 6. Dega H, Robert J, Bonnafous P, et al. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother 2000; 44: 2367-2372.
- 7. Portaels F, Traore H, De Ridder K, Meyers WM. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob Agents Chemother 1998; 42: 2070-2073.
- 8. Ross BC, Marino L, Oppedisano F, et al. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol 1997; 35: 1696-1700.




