|
Research
Iodine deficiency in ambulatory participants at a Sydney teaching
hospital: is Australia truly iodine replete?
Jenny E Gunton, Graham Hams, Marcelle Fiegert and Aidan McElduff
MJA 1999; 171: 467-470
For editorial comment, see Eastman
Abstract -
Introduction -
Methods -
Results -
Discussion -
References -
Authors' details
-
-
More articles on Endocrinology
|
| |
Abstract |
Objective: To assess iodine status in four separate
groups -- pregnant women, postpartum women, patients with diabetes
mellitus and volunteers.
Design and setting: Prospective cross-sectional
study at a tertiary referral hospital in Sydney.
Participants: 81 pregnant women attending a "high
risk" obstetric clinic; 26 of these same women who attended three
months postpartum; 135 consecutive patients with diabetes mellitus
attending the diabetes clinic for an annual complications screen;
and 19 volunteers. There were no exclusion criteria.
Methods: Spot urine samples were obtained, and
urinary iodine was measured by inductively coupled plasma mass
spectrometer.
Outcome measures: Iodine status based on urinary
iodine concentration categorised as normal (> 100 µg/L), mild
deficiency (51-100 µg/L) and moderate to severe deficiency (< 50
µg/L).
Results: Moderate to severe iodine deficiency was
found in 16 pregnant women (19.8%), five postpartum women (19.2%), 46
patients with diabetes (34.1%) and five volunteers (26.3%). Mild
iodine deficiency was found in an additional 24 pregnant women
(29.6%), nine postpartum women (34.6%), 51 patients with diabetes
(37.8%) and 9 normal volunteers (47.4%). Median urinary iodine
concentration was 104 µg/L in pregnant women, 79 µg/L in postpartum
women, 65 µg/L in patients with diabetes mellitus and 64 µg/L in
volunteers.
Conclusions: The high frequency of iodine
deficiency found in our participants suggests that dietary sources
of iodine in this country may no longer be sufficient. Further
population studies are required.
|
| | Introduction |
It is currently believed that iodine deficiency does not exist in
Australia.1,2 However, iodine status is
seldom, if ever, measured in routine clinical care, and iodine
deficiency may have significant adverse consequences,
particularly during pregnancy (Box 1). Box 2 shows some of the reasons
why iodine intake in Australia may be inadequate.
The recommended daily intake (RDI) of iodine is 100 µg daily for the
general population and 150-200 µg daily for women who are pregnant or
breastfeeding6-8,10 (iodine demand
increases during pregnancy because of increased renal clearance and
fetal iodine transfer).
Approximately 90% of iodine is excreted in the urine,1,11 and iodine
status is usually assessed by measuring urinary iodine
concentration.
The accepted minimum adequate level of urinary iodine is 100 µg/L, and
levels above this are considered normal.1,6-9,12 Urinary iodine
concentrations below 25 µg/L are classified as severe deficiency,
and are associated with an increased risk of cretinism; 26-50 µg/L is
classified as moderate deficiency, and 51-100 µg/L is regarded as
mild iodine deficiency.1,6-9,12 The World Health
Organization (WHO) recommends that the median urinary iodine
concentration for populations as a whole should be more than 100 µg/L,
that less than 20% of the population should have a urinary iodine
concentration below 50 µg/L, and that no cretinism
occurs.12
Having previously found low levels of free thyroxine in pregnant
women,13 and in light of the adverse
consequences of iodine deficiency during pregnancy, we initially
set out to test the iodine status of a group of pregnant women. We
subsequently included other groups to widen our investigation of
iodine status.
|
| |
Methods |
| |
Study participants | |
Our study was conducted at a tertiary referral hospital in Sydney.
Participants in the study included women who attended a specialist
"high risk" obstetric clinic, patients of both sexes with diabetes
who attended the hospital's diabetes clinic, and healthy,
non-pregnant volunteers recruited after a presentation about
iodine.
Participants thus comprised 81 consecutive pregnant women who
attended the obstetric clinic between 1 August 1998 and 1 April 1999,
26 of these same women who were reassessed at three months postpartum,
135 consecutive patients who attended the diabetes clinic for an
annual complications screen between 1 November 1998 and 1 February
1999, and 19 volunteers recruited between 1 February and 1 July 1999.
All participants provided a routine urine sample. There were no
exclusion criteria.
One of the 81 pregnant women had thyrotoxicosis as a result of Graves'
disease -- she particpated before commencing therapy. Twenty-two of
the patients attending the diabetes clinic (16.1%) had type 1
diabetes, 103 (76.3%) had type 2 diabetes and 10 (7.5%) had impaired
glucose tolerance.
One of the patients with diabetes had recently received
iodine-containing intravenous contrast medium during a coronary
angiogram, and one was taking amiodarone. No other participant was
known to have received contrast medium, or to be taking amiodarone or
iodine supplements.
| |
Urinary iodine measurement | |
Urinary iodine concentrations were determined by means of a Varian
UltraMass inductively coupled plasma mass spectrometer with SPS-5
autosampler (Varian Inc., Palo Alto, California, USA). The
measurement was calibrated over a range of 0-1000 µg iodine per litre.
The lower limit of detection for the assay was 2 µg/L. The
reproducibility of the assay as represented by the 100 µg/L
calibrator assessed over three months was ± 6 µg/L (± 2 SD). Comparison
with the colorimetric/Sandell-Koltkoff reaction method showed a
highly significant correlation (P < 0.001; see Box 3).
Other authors have also compared the methods and found high
correlation.14 In particular, no
systematic biases were found at low iodine concentrations.
Some investigators use the urinary iodine/creatinine ratio to
determine iodine status.1,14 We thus measured urinary
creatinine by the Creatinine Jaffa method (Boehringer Mannheim
Systems, Mannheim, Germany) and calculated iodine/creatinine
ratios (µg iodine/g creatinine) for each participant. The
correlation between urinary iodine and iodine/creatinine ratio was
high for non-pregnant participants (r = 0.969; P <
0.001) and lower for the pregnant group (r = 0.419; P
< 0.001).
Twenty-four-hour urinary iodine measurement may be used to assess
iodine status,6 but this method can be
unreliable because of incorrect or incomplete
collection,15 and is less practical than
spot samples for population surveys.9,12 To compare this method
with our spot sampling, we selected six pregnant women (on the basis of
their spot urine concentrations to cover a range of values) who
collected 24-hour urine samples for iodine content measurement. The
correlation was highly significant (r2 = 0.82), thus
confirming that spot urine samples were a reliable way of measuring
iodine status.
As part of routine care, 70 of the 81 pregnant women and 121 of the 135
patients with diabetes had thyroid function tests. Free thyroxine
(FT4) and thyroid-stimulating hormone (TSH) levels
were measured by means of an automated chemiluminescence system
(Chiron Diagnostics, Scoresby, Vic.).
We did not seek ethical approval for this study as it involved no
deviation from usual care, except in the case of the 19 volunteers who
agreed to provide a urine sample.
| |
Statistical analysis | |
We used SPSS for statistical analysis.16 Means are expressed with ±
2 standard deviations, and medians with 95% confidence intervals
(CI) are shown where data were not normally distributed. The results
of non-parametric variables (including iodine results) were
compared by means of the Mann-Whitney Wilcoxon rank sum test.
|
| |
Results | |
Box 4 shows the mean and median ages and the results of spot urinary
iodine concentration for the four groups. The three non-pregnant
groups had similar urinary iodine concentration results, with a
slightly higher median in the postpartum group compared with the
group with diabetes. As expected,17-19 the pregnant women had
higher urinary iodine concentrations than the other groups as a whole
(P = 0.004). The iodine/creatinine ratios also show a high
proportion of abnormal results (Box 4).
The median iodine concentration in the 26 postpartum women (79 µg/L)
who provided repeat urine samples for iodine measurement three
months after delivery was considerably lower than that in the 81
pregnant women (104 µg/L). However, this difference was not
statistically significant (P = 0.249).
The patient with diabetes who had received iodine-containing
intravenous contrast medium during a coronary angiogram in the month
before the urinary spot test had a urinary iodine concentration of
2170 µg/L.
Box 5 shows TSH levels and FT4 levels versus iodine status
in pregnant and non-pregnant participants. There was no significant
relationship between iodine status and FT4 or TSH levels
in either the pregnant group or non-pregnant group. Separate
analysis of patients with diabetes and postpartum women did not
significantly alter these results. However, there was a weak
correlation between FT4 and urinary iodine levels when
examined as a continuous variable (Pearson correlation
coefficient, 0.26; P = 0.016).
|
| |
Discussion | |
By WHO criteria,12 the median iodine levels
in our pregnant participants were only just adequate, while those in
postpartum women, patients with diabetes and normal volunteers were
inadequate. The slightly higher median iodine level in the
postpartum group compared with that in the group with diabetes may
have been the result of this concentration not having returned to
baseline after pregnancy, although further study is required to
document the rate of change post partum. We believe the low
values in patients with diabetes was not a problem specific to
diabetes, but merely a reflection of low urinary iodine levels in the
general population. The similarity between the patients with
diabetes and our small group of volunteers supports this view. Our
data are consistent with generally low iodine intake.
Our findings mirror recent reports from other countries.9,11,20 A United
States study showed that the median urinary iodine concentration in
1988-1994 had decreased by more than 50% from that in
1971-1974.9,11 The 1988-1994 results
showed 11.7% of the US population to be iodine deficient (a 4.5-fold
increase since 1971-1974). The mean urinary iodine concentration in
that population was 265 µg/L, and people from higher socioeconomic
groups were more likely to be iodine deficient. Our data may also
reflect this effect, as, although our patients were attending a
public clinic, the hospital catchment area is a relatively high
socioeconomic group. Our data suggest that Australia may be
experiencing a similar trend to that seen in the US.
Iodine deficiency during pregnancy can affect the thyroid glands of
both the mother and baby,10,17-19 and may have many
adverse health consequences (Box 1). Some, but not all, researchers
have found an increase in urinary iodine levels during
pregnancy.10,17-19 Smyth et al studied
urinary iodine concentration in a group of pregnant women in an area of
Ireland with known borderline iodine deficiency.10 In the third
trimester, they found a mean urinary iodine concentration of 132 µg/L
(standard error of the mean, 6.8), and found that urinary iodine
concentration increased during pregnancy. In a more
iodine-deficient area, Glinoer et al found that urinary iodine
concentration did not increase during pregnancy (median iodine
concentration 45 µg/L after 20 weeks' gestation, no mean
given).19 So, it is not clear whether
the apparently higher levels in our pregnant women were pregnancy
related or, in fact, masked iodine deficiency in pregnancy.
We found that a considerable percentage of pregnant women (4.9%) were
severely iodine deficient, with spot urine results of < 25 µg/L.
While this is the threshold below which cretinism may occur, other
factors, such as selenium deficiency and the presence of dietary
goitrogens, also play a part in determining cretinism,21 and these two
factors are not usually seen in Sydney. Therefore, we would not expect
to see an increased incidence of cretinism in Sydney on the basis of
these results alone. However, more subtle adverse fetal outcomes may
occur.
Our findings suggest that we should no longer automatically consider
Australia an iodine-replete country. We found that iodine
deficiency was common among 235 people attending a Sydney teaching
hospital and speculate that these data are applicable to the general
population, although this will require independent confirmation.
The frequency of iodine deficiency in our pregnant population
(18.8%) approaches the maximum acceptable level recommended by WHO
(20%); this recommendation was exceeded in our group with diabetes
(34.1%) and the normal volunteers (26.3%). The postpartum women had a
median iodine concentration of 79 µg/L, which is lower than the WHO
recommendation of 100 µg/L. This has important public health
implications.
The weaknesses of this study include the small group of normal
volunteers, and perhaps the use of a sample from a teaching hospital
rather than the community. The normal volunteers had results which
are equivalent to those seen in postpartum women and non-pregnant
patients with diabetes. Our subjects were all ambulatory, not
inpatients at the time of testing, and generally well. Although
24-hour urinary iodine excretion studies may be the ideal method of
assessing iodine status, these are not generally performed in large
numbers for a variety of technical and practical reasons.
A weak correlation between urinary iodine and free thyroxine was
observed for all non-pregnant participants in total, and for the
participants with diabetes mellitus. Because of the large number of
other factors which influence thyroid function (including
pregnancy),13 the relatively loose
correlations are an expected finding.
Further studies are needed, and these include (i) population surveys
in Sydney and elsewhere in Australia; (ii) assessment of thyroid size
(eg, by ultrasound) in relation to iodine status; and (iii) detailed
assessment of neonates, including thyroid size, neonatal TSH
levels, and detailed neurological outcomes.
|
| |
References | |
- Hetzel BS. Iodine deficiency disorders. In: Garrow JS, James WPT,
editors. Human nutrition and dietetics. Edinburgh: Churchill
Livingstone, 1993: 534-555.
-
Mortimer RH. Thyroid disease and pregnancy. Aust N Z J Med
1998; 28: 647-653.
-
Tasmanian Thyroid Advisory Committee. Study in disease
surveillance. 1950-1979. Med J Aust 1981; 2: 234-238.
-
Clements FW. Goitre studies. 1. The incidence of endemic goitre in
three areas in Australia. Med J Aust 1948; 21: 637-639.
-
Hales I. Studies in diseases of the thyroid gland [MD thesis]
Sydney: University of Sydney, 1971.
-
Boyages S. Iodine deficiency disorders. J Clin Endocrinol
Metab 1993; 77: 587-591.
-
Clugston GA, Hetzel BS. Iodine. In: Shils ME, Olson JA, Shike M,
editors. Modern nutrition in health and disease. 8th ed. Vol. 1.
Philadelphia: Lea and Febiger, 1994; 252-263.
-
Delange F. The disorders induced by iodine deficiency.
Thyroid 1994; 4: 107-128.
-
Hollowell JG, Staehling NW, Hannon WH, et al. Iodine nutrition in
the United States. Trends and public health implications: iodine
excretion data from the National Health and Nutrition Examination
Surveys I and III (1971-1974 and 1988-1994). J Clin Endocrinol
Metab 1998; 83: 3401-3408.
-
Smyth PPA, Hetherton AMT, Smith DF, et al. Maternal iodine status
and thyroid volume during pregnancy: correlation with neonatal
iodine intake. J Clin Endocrinol Metab 1997; 82: 2840-2843.
-
Dunn JT. What's happening to our iodine? [editorial]. J Clin
Endocrinol Metab 1998; 83: 3398-3400.
-
World Health Organization Nutrition Unit. Indicators for
assessing iodine deficiency disorders and their control through
salt iodization. Document No. WHO/NUT 94.6. Geneva: WHO, 1994: 36.
-
McElduff A. Measurement of free thyroxine levels
(fT4) in pregnancy. Aust N Z J Obstet Gynaecol
1999; 39: 158-161.
-
May SL, May WA, Bourdoux PP, et al. Validation of a simple, manual
urinary iodine method for estimating the prevalence of
iodine-deficiency disorders, and interlaboratory comparison with
other methods. Am J Clin Nutr 1997; 65: 1441-1445.
-
McElduff A, Shuter B, Cooper R, et al. Measuring renal function in
patients with diabetes mellitus. J Diabetes Complications
1997; 11: 225-229.
-
SPSS [computer program], version 6.0. Chicago, Ill: SPSS
Inc, 1996.
-
Silva JE, Silva S. Interrelationships among serum thyroxine,
triiodothyronine, reverse triiodothyronine, and
thyroid-stimulating hormone in iodine-deficient pregnant women
and their offspring: effects of iodine supplementation. J Clin
Endocrinol Metab 1981; 52: 671-677.
-
Glinoer D, De Nayer P, Bourdoux et al. Regulation of maternal
thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:
276-287.
-
Glinoer D, Delange F, Laboureur I, et al. Maternal and neonatal
thyroid function at birth in an area of marginally low iodine intake.
J Clin Endocrinol Metab 1992; 75: 800-805.
-
Valiex P, Zarabska M, Preziosi P, et al. Iodine deficiency in
France [letter]. Lancet 1999; 353: 1766-1767.
-
Moreno-Reyes R, Suetens C, Mathieu F, et al. Kashin-Beck
osteoarthropathy in rural Tibet in relation to selenium and iodine
status. N Engl J Med 1998; 339: 1112-1120.
Received 13 Apr, accepted 21 Aug, 1999
|
| | Authors' details |
Royal North Shore Hospital, St Leonards, NSW.
Jenny E Gunton, MB BS, Endocrine Fellow, Department of
Endocrinology.
Graham Hams, MAppSc, Senior Staff
Scientist, Pacific Laboratory Medicine Services.
Marcelle
Fiegert, BEd, MNutri Diet, Dietitian, Department of Nutrition.
Aidan McElduff, FRACP, PhD, Senior Staff Specialist in
Endocrinology, Department of Endocrinology.
Reprints: Dr J E Gunton, C/- Clinic 1, Royal North Shore
Hospital, St Leonards, NSW 2065.
jennyegAThotmail.com.
|
| | |
1: Iodine deficiency disorders
Maternal
- Goitre
- Hypothyroidism
- Decreased fertility
- Miscarriage
Fetal
Neonatal
- Cretinism
- Increased mortality
- Goitre
- Hypothyroidism
|
|
| | Back to text | |
| |
2: The iodine situation in Australia- In the past, an increased incidence of goitre and iodine deficiency was documented in certain parts of Australia.3-5
- Prevention of iodine deficiency in industrialised countries most commonly relies on iodised salt, iodine in milk, or iodine-supplemented bread.1,6-8
- The upper limit of the recommended daily intake of salt (NaCl) is 100 mmol, or 6 g (a heaped teaspoon); 100 mmol of iodised salt per day would provide 175-240 µg of iodine. However, most salt is incorporated into foods before purchase, and the three major Australian manufacturers of processed food we contacted all reported using non-iodised salt only.
- Non-iodised table salt is readily available, and may be used more frequently than in the past as campaigns to use iodised salt are forgotten. (We reviewed supermarket shelves in our local area, and found that the space allocated for display suggests that more non-iodised than iodised salt is purchased.)
- In the United States, only 50%-60% of salt currently consumed is iodised.9
- Milk products, which used to contain significant concentrations of iodine (up to 300 µg/100 mL) by virtue of iodine-containing solutions used to clean the milk vats, now contain low levels of iodine because volatile cleaning solutions are used (Dairy Farmers Association, Nutrition Panel for Milks, personal communication).
- While the incidence of iodine deficiency and goitre was decreased by legislation requiring iodine supplementation of bread in 1966,3 this is no longer a requirement (because of concerns about an increased incidence of thyrotoxicosis).
- Marine fish, shellfish, seaweed and kelp contain high amounts of iodine,1,7 and such ocean seafood, as well as added iodised salt, provide most of the iodine in the Australian diet. However, many people may consume these products rarely, if at all.
|
| | Back to text | | 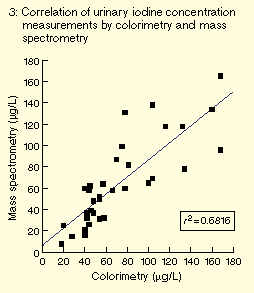
| | Back to text | |
4: Iodine status results | | Group | Pregnant women | Postpartum women | Patients with diabetes | Volunteers |
| | Number of participants | 81 | 26 | 135 | 19 | | Age (years) | | Mean (± 2 SD) | 32.9 ± 9.8 | 35.3 ± 11.3 | 50.1 ± 35.3* | 49.5 ± 17.4* | | Median (95% CI) | 34 (24-42) | 35 (25-42) | 50 (25.7-83.0) | 49 (45.3-53.8) | | Spot iodine concentration (µg/L) | | Median | 104 | 79‡ | 65† | 64 | | (95% CI) | (89-129) | (44-229) | (58-89) | (54-75) | | No. of participants (%) with | | Severe to moderate deficiency < 50 µg/L | 16 (19.8%) | 5 (19.2%) | 46 (34.1%) | 5 (26.3%) | | Mild deficiency 51-100 µg/L | 24 (29.6%) | 9 (34.6%) | 51 (37.8%) | 9 (47.4%) | | Normal iodine status > 100 µg/L | 41 (50.6%) | 12 (46.1%) | 38 (28.1%) | 5 (26.3%) | | Iodine/creatinine ratio (µg iodine/g creatinine) | | Median | 159 | 131 | 114 | 108 | | (95% CI) | (169-232) | (106-218) | (93-505) | (84-209) | | No. of participants (%) with | | Severe to moderate deficiency | | < 50 µg iodine/g creatinine | 6 (7.4%) | 2 (7.7%) | 7 (5.2%) | 1 (5.3%) | | Mild deficiency 51-100 µg iodine/g creatinine | 22 (27.2%) | 9 (34.6%) | 50 (37.0%) | 5 (26.3%) | | Normal iodine status > 100 µg iodine/g creatinine | 53 (65.4%) | 15 (57.7%) | 78 (57.8%) | 13 (68.4%) |
| | * P < 0.001 for comparison with pregnant women. †P < 0.01 for comparison with pregnant women.
‡P < 0.05 for comparison with patients with diabetes.
|
| | Back to text | | | | Back to text |
|





